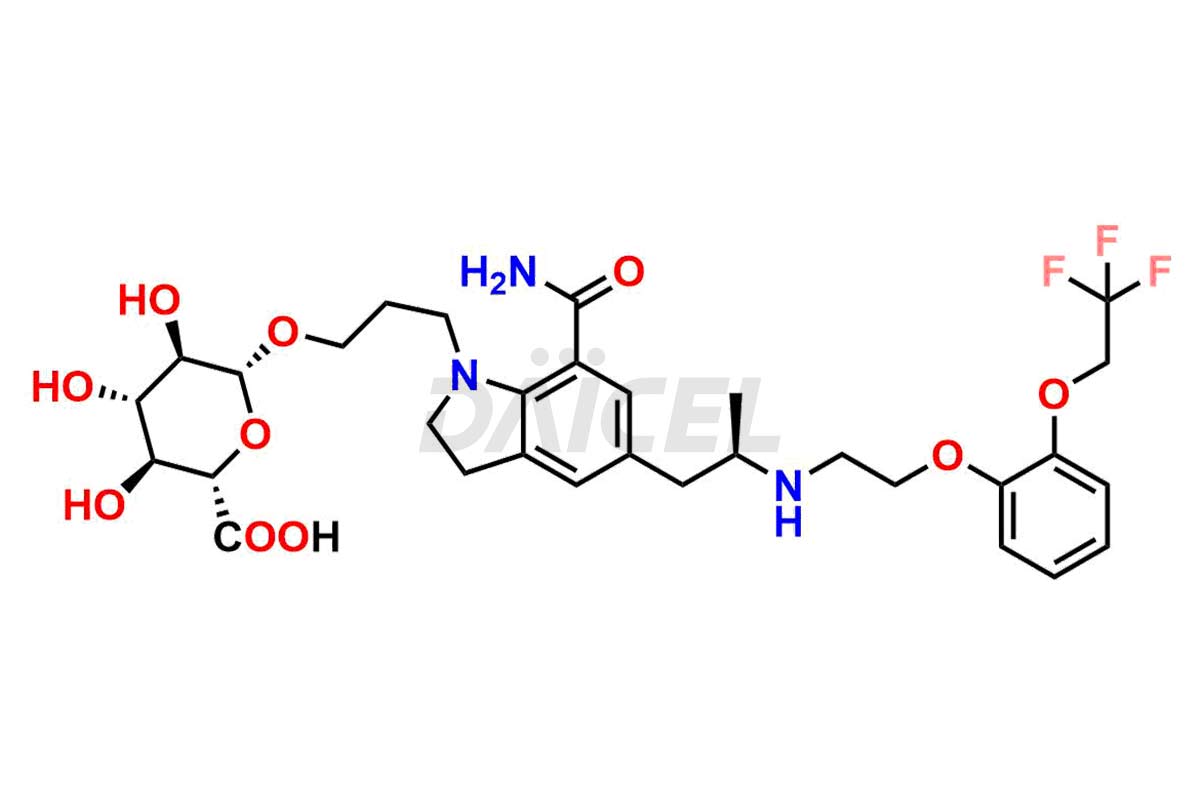
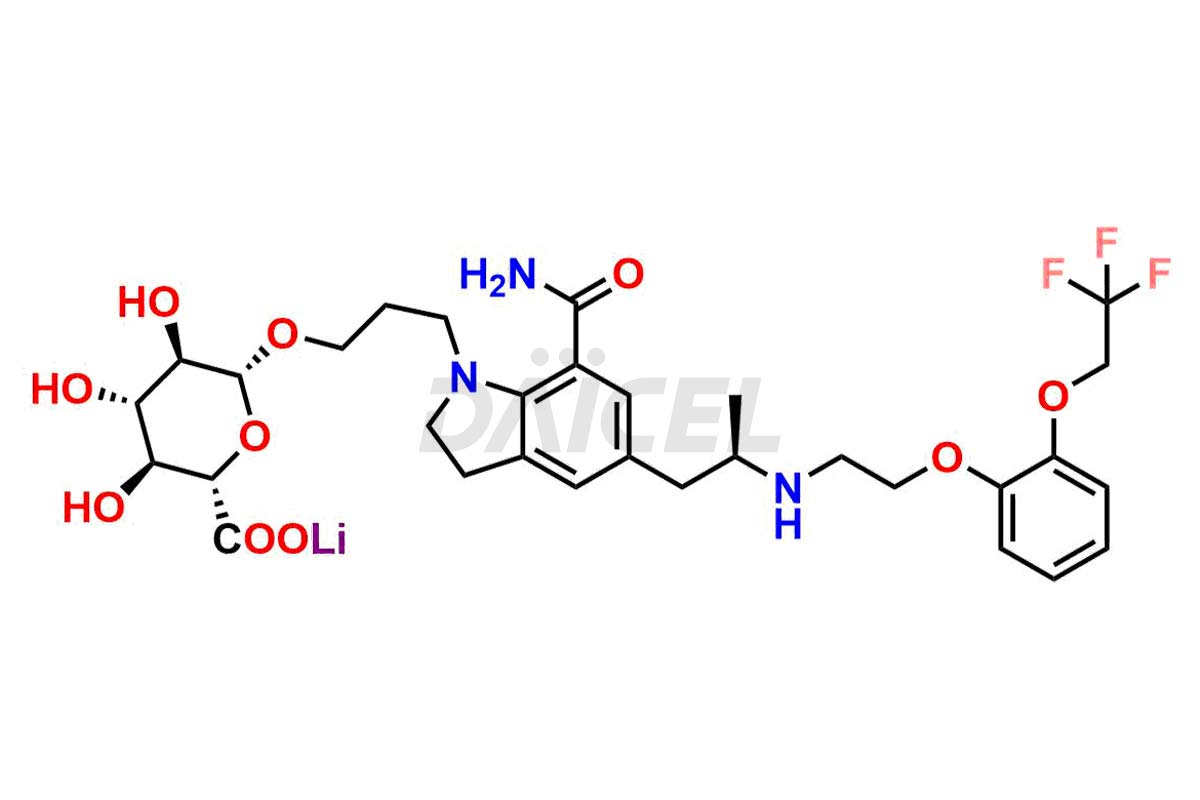
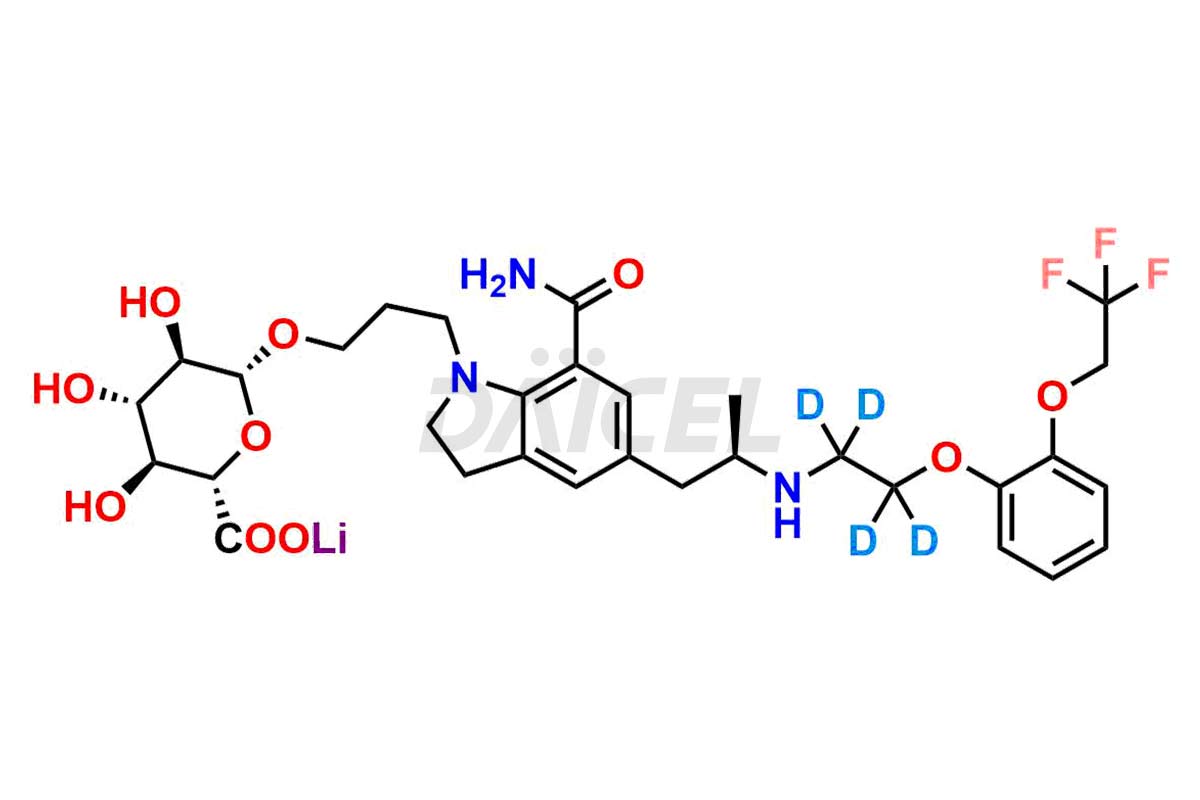
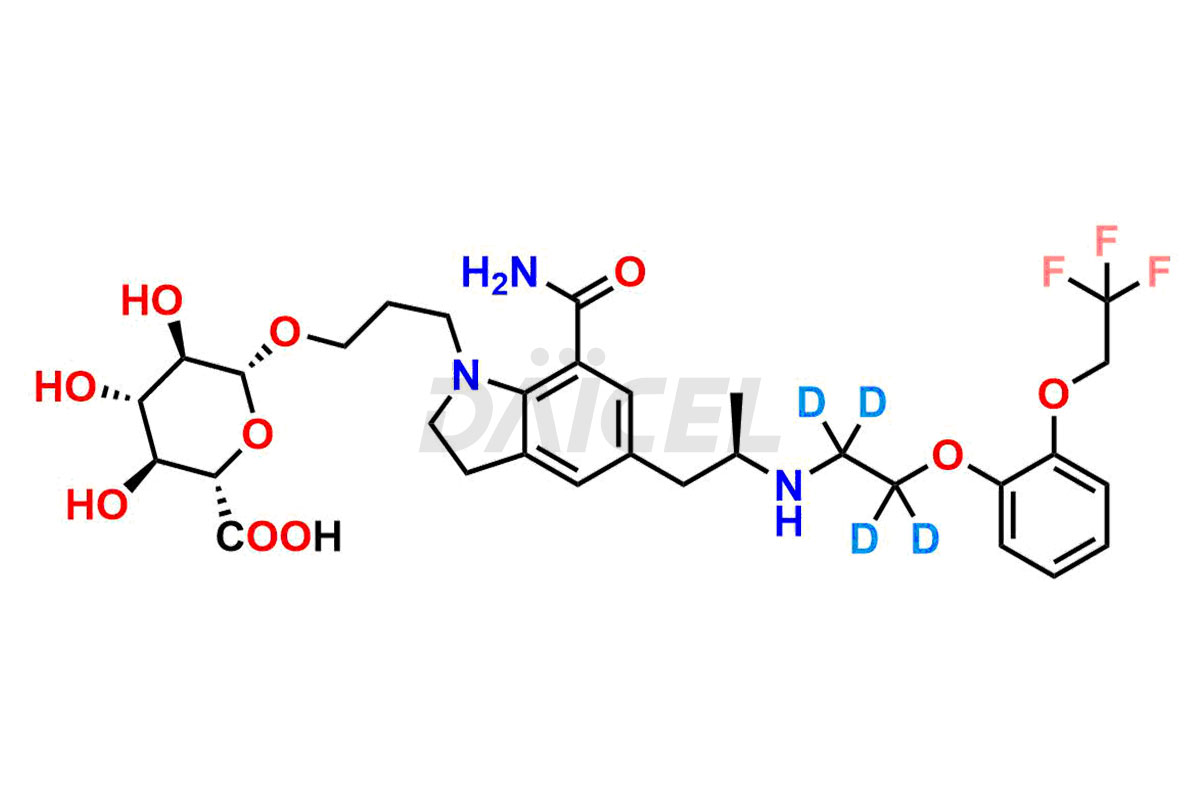
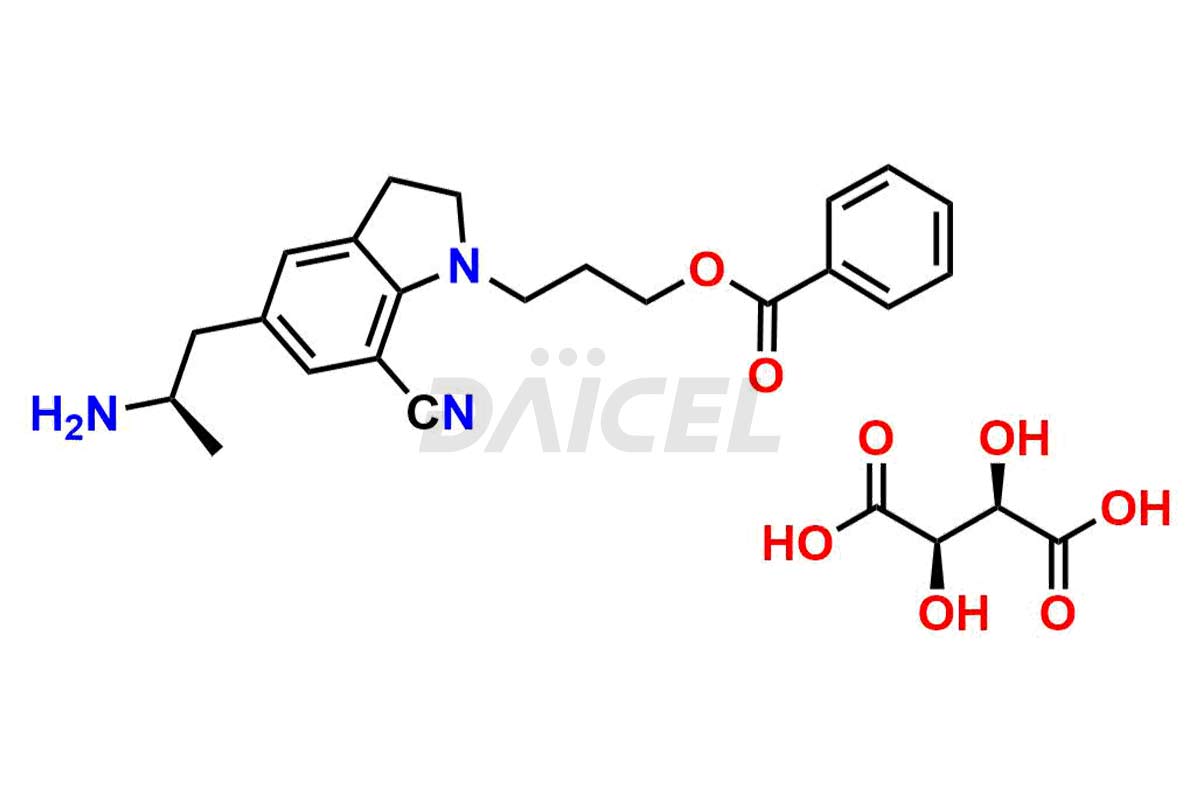
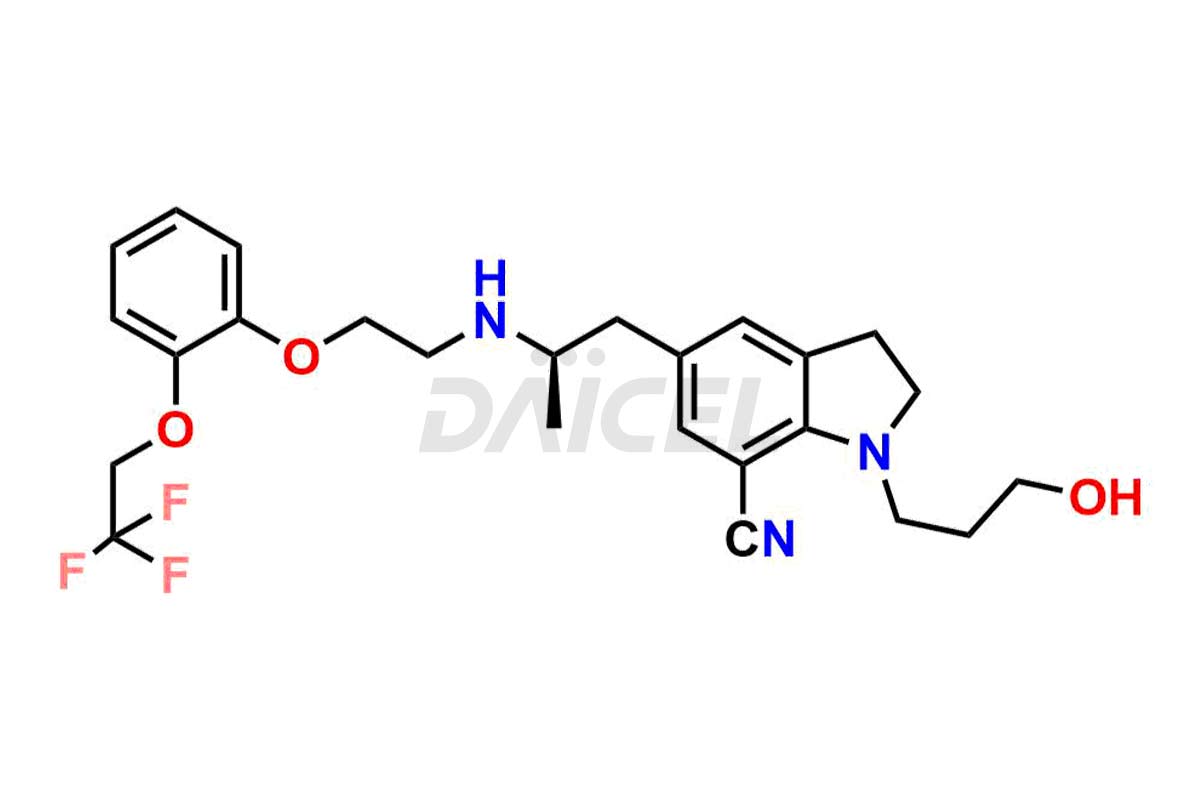
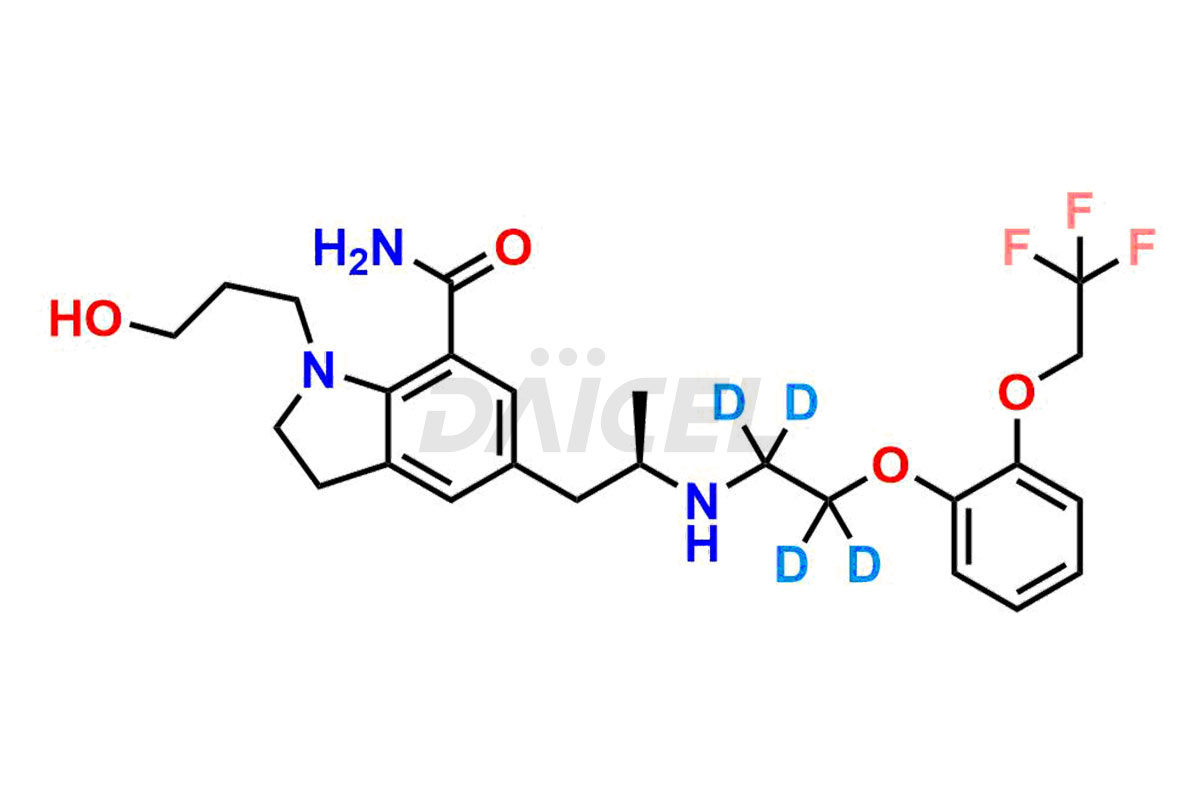
Silodosin
General Information
Silodosin Impurities and Silodosin
Daicel Pharma is a reliable source for synthesizing high-quality Silodosin impurities, like
Silodosin Dehydro Impurity, Silodosin Glucuronide -Li salt, Silodosin Nitrile Impurity, etc. These impurities are essential for accurate analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Silodosin. Additionally, Daicel Pharma specializes in the custom synthesis of Silodosin impurities, catering to specific client requirements. These high-quality impurities can be shipped globally, offering convenience and flexibility to customers worldwide.
Silodosin [CAS: 160970-54-7] is a selective alpha-1a adrenergic antagonist that treats the symptoms of benign prostatic hyperplasia (BPH).
Silodosin: Use and Commercial Availability
Silodosin is a medication primarily used to treat benign prostatic hyperplasia (BPH) symptoms. It helps to improve urine flow and reduce symptoms such as difficulty urinating, weak urine flow, frequent urination, and the urge to urinate urgently. Silodosin helps relieve lower urinary tract symptoms (LUTS) associated with BPH.
Silodosin is available under the brand Rapaflo, which contains the active ingredient, Silodosin.
Silodosin Structure and Mechanism of Action 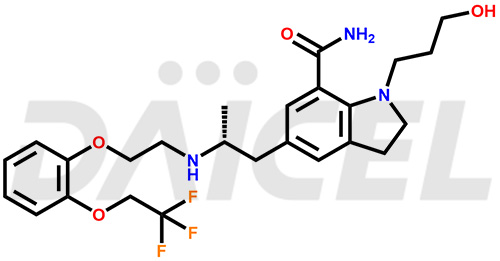
The chemical name of Silodosin is 2,3-Dihydro-1-(3-hydroxypropyl)-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-1H-indole-7-carboxamide. Its chemical formula is C25H32F3N3O4, and its molecular weight is approximately 495.5 g/mol.
Silodosin blocks post-synaptic alpha-1 adrenoreceptors causing smooth muscles in the human prostate, bladder base, bladder neck, urethra, and prostatic capsule to relax. It improves urine flow and reduces BPH symptoms.
Silodosin Impurities and Synthesis
During Silodosin’s manufacturing1, impurities formation is possible and can compromise its effectiveness. These impurities can arise from various sources, including raw materials, intermediates, and chemicals to synthesize Silodosin. Closely managing and monitoring these impurities is paramount to ensure the drug’s optimal efficacy and safety.
Daicel Pharma offers a Certificate of Analysis (CoA) for Silodosin impurity standards, encompassing impurities such as Silodosin Dehydro Impurity, Silodosin Glucuronide -Li salt, Silodosin Nitrile Impurity, among many others. The CoA provides detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, we give a detailed 13C-DEPT on delivery. With advanced technology and expertise, Daicel can synthesize any unknown Silodosin impurity or degradation product. We also synthesize labeled compounds. Daicel offers deuterium-labeled standards, Silodosin glucuronide-D4, Silodosin-D4, and Silodosin Glucoronide D4 -Li salt for bioanalytical research and BA/BE studies.
References
FAQ's
References
- Kitazawa, Makio; Ban, Masaaki; Okazaki, Kosuke; Ozawa, Motoyasu; Yazaki, Toshikazu; Yamagishi, Ryoichi, Indoline compounds for the treatment of dysuria, Kissei Pharmaceutical Co., Ltd., Japan, EP600675, July 8, 1998
- Zhao, Xia; Liu, Yuwang; Xu, Junyu; Zhang, Dan; Zhou, Ying; Gu, Jingkai; Cui, Yimin, Determination of silodosin in human plasma by liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 877, Issue: 29, Pages: 3724-3728, 2009
Frequently Asked Questions
Why is the presence of impurities a concern in Silodosin?
Impurities in Silodosin can affect its quality, safety, and efficacy. Depending on the type and level of impurities, they can impact the drug's pharmacological activity and stability and pose potential risks to patient health.
How are impurities in Silodosin detected and quantified?
Impurities in Silodosin are detected and quantified using reversed-phase (RP) ultra-high-performance liquid chromatography (UHPLC). This method will allow for accurate identification and quantification of impurities.
What steps can be taken to control impurity levels in Silodosin during manufacturing?
Manufacturers can adopt several strategies to maintain control over impurity levels in Silodosin. They include using high-quality starting materials, optimizing synthesis and purification processes, implementing comprehensive quality control tests, and monitoring impurity levels at different stages of the manufacturing process.
What are the temperature conditions required to store Silodosin Impurities?
Silodosin Impurities are stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.