LOAD MORE
You're viewed 9 of 10 products
Daicel Pharma synthesizes high-quality impurities of Semaglutide, including Des-Gly (10)-Semaglutide, D-Ser(14)-Semaglutide, and Des- His(7)-Semaglutide, which are valuable in evaluating the quality, stability, and biological safety of the active pharmaceutical ingredient Semaglutide. Moreover, Daicel Pharma offers custom synthesis of Semaglutide impurities for global delivery.
Semaglutide [CAS: 910463-68-2] is a polypeptide composed of a linear sequence of 31 amino acids linked together by peptide bonds. It’s a long-lasting analog glucagon-like peptide (GLP-1). It helps in the management of type 2 diabetes in adults.
Semaglutide is a medicine to treat adults with uncontrollable type 2 diabetes mellitus. It can be used as a standalone treatment when metformin is considered inappropriate due to intolerance or contradictions or in addition to other diabetes medicines. Semaglutide is indicated as a supplement to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Further, it helps to reduce the risk of cardiovascular events in some populations.
Novo Nordisk launched Semaglutide as Ozempic (subcutaneous injection) and Rybelsus (oral tablets) in the market. Ozempic and Rybelsus have both received approval from regulatory authorities such as USFDA, Health Canada, European Medicines Agency, and the Japanese Health Ministry. Further, Novo’s Wegovy (Semaglutide injection) is indicated for weight management in adults with a BMI of 30 kg/m² or greater (obesity) or 27 kg/m² or greater (overweight) with at least one weight-related comorbidity, such as hypertension, dyslipidemia, etc.
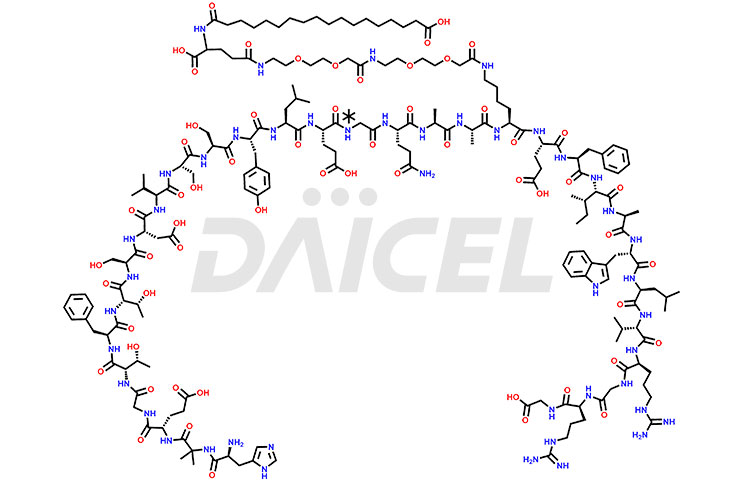
The chemical formula for Semaglutide is C187H291N45O59, and its molecular weight is approximately 4113.58 g/mol.
Semaglutide acts as a GLP-1 receptor antagonist that binds and activates the GLP-1 receptor. It is stabilized against degradation by the DPP-4 enzyme. Semaglutide reduces blood glucose, stimulates insulin secretion, and lowers glucagon secretion in a glucose-dependent manner.
The process for preparing Semaglutide involves a fermentation process in yeast cells. Impurities such as D-amino acid impurities, D-Ser, D-His, D-Asp degradants, etc., may form during the production1,2 and storage of Semaglutide. The presence of these impurities in the drug compromises its efficacy, safety, and stability. And so, the manufacturing process of Semaglutide needs to be optimized to minimize the formation of such impurities.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Semaglutide impurity standards3, including Des-Gly (10)- Semaglutide, Des-His(7)-Semaglutide, and D-Ser(14)-Semaglutide. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity4. We also provide 13C-DEPT and CHN upon request. In addition, we provide, on request complete characterization report.
Daicel has the technology and expertise to prepare any unknown Semaglutide impurity or degradation product. We also provide labeled compounds to quantify the efficacy of generic Semaglutide. Daicel offers Semaglutide D5, and Semaglutide D8, deuterium-labeled standards of Semaglutide for bioanalytical research and BA/BE studies with isotope data in CoA.
The process-related impurities of Semaglutide include peptide impurities like Endo-Gly(4), Endo-Gly(16), Endo-Gly(31), Endo-Ala(18), D-Ser(11), D-Ser(12), and inorganic impurities, which can affect the drug’s safety, quality, and efficacy.
Semaglutide impurities can affect the shelf life of the drug product due to the degradation of the active pharmaceutical ingredient or reaction with other components during formulation.
Liquid chromatography-mass spectrometry (LC-MS), infrared (IR) spectroscopy, and high-performance liquid chromatography (HPLC) are some of the analytical methods used in the identification & characterization processes of Semaglutide impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.