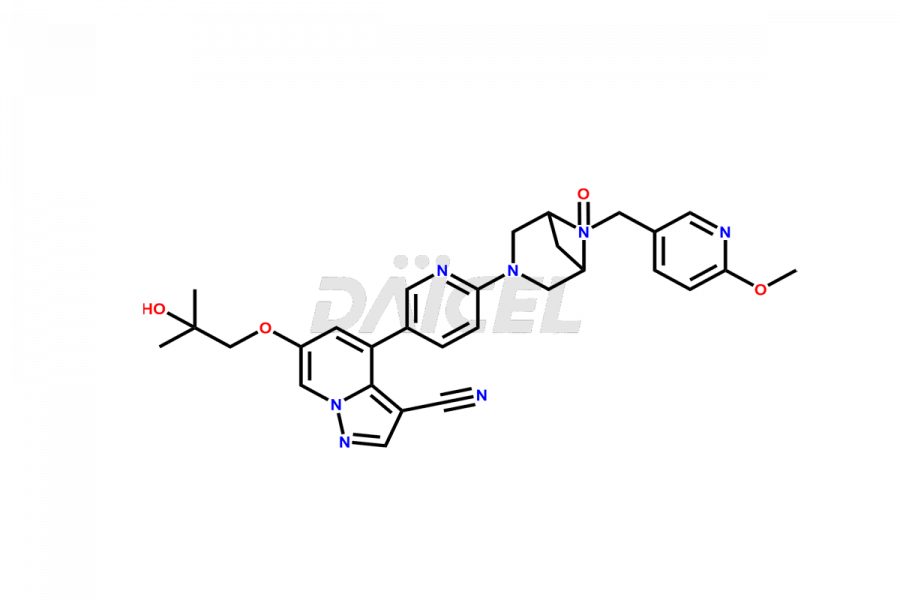
Selpercatinib
General Information
Selpercatinib Impurities and Selpercatinib
Daicel Pharma is a trusted provider of quality Selpercatinib impurities, including Selpercatinib Amide Impurity and Selpercatinib Metabolite M2. These impurities help in evaluating active pharmaceutical ingredients’ quality, stability, and safety. Furthermore, Daicel Pharma specializes in custom synthesis of Selpercatinib impurity standards, guaranteeing to meet individual client specifications. With global shipping capabilities, these impurities can be conveniently delivered to customers worldwide, offering unparalleled convenience.
Selpercatinib [CAS: 2152628-33-4] is a highly-selective RET kinase inhibitor with central nervous system (CNS) activity. It treats RET fusion-positive lung and thyroid cancers.
Selpercatinib: Use and Commercial Availability
Selpercatinib is a potent and selective RET kinase inhibitor to treat specific types of cancers. It treats patients with advanced or metastatic RET fusion-positive non-small cell lung cancer (NSCLC), RET-mutant medullary thyroid cancer (MTC), and RET fusion-positive thyroid cancer. Selpercatinib is known to effectively inhibit the abnormal RET signaling pathway, which plays a crucial role in the development and progression of these malignancies.
Selpercatinib is available under Retevmo.
Selpercatinib Structure and Mechanism of Action 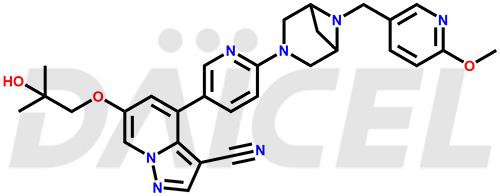
The chemical name of Selpercatinib is 6-(2-Hydroxy-2-methylpropoxy)-4-[6-[6-[(6-methoxy-3-pyridinyl)methyl]-3,6-diazabicyclo[3.1.1]hept-3-yl]-3-pyridinyl]pyrazolo[1,5-a]pyridine-3-carbonitrile. Its chemical formula is C29H31N7O3, and its molecular weight is approximately 525.6 g/mol
Selpercatinib inhibits wild-type, Rearranged during Transfection (RET), required for the nervous system and kidney development.
Selpercatinib Impurities and Synthesis
Selpercatinib impurities can arise during their synthesis1 due to storage or using specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Selpercatinib for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Selpercatinib impurity standards such as Selpercatinib Amide Impurity and Selpercatinib Metabolite M2. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, we give a complete 13C-DEPT. Daicel Pharma possesses the technology and expertise to synthesize any unknown Selpercatinib impurity or degradation product.
References
FAQ's
References
- Andrews, Steven W.; Aronow, Sean; Blake, James F.; Brandhuber, Barbara J.; Cook, Adam; Haas, Julia; Jiang, Yutong; Kolakowski, Gabrielle R.; McFaddin, Elizabeth A.; McKenney, Megan L.; et al, Substituted Pyrazolo[1,5-A]Pyridine Compounds As RET Kinase Inhibitors, Array Biopharma Inc, EP3523301B1, May 27, 2020
- Senturk, Rahime; Wang, Yaogeng; Schinkel, Alfred H.; Beijnen, Jos H.; Sparidans, Rolf W., Quantitative bioanalytical assay for the selective RET inhibitors selpercatinib and pralsetinib in mouse plasma and tissue homogenates using liquid chromatography-tandem mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 1147, Pages: 122131, 2020
Frequently Asked Questions
Do its impurities impact the pharmacokinetics of Selpercatinib?
The impurities present in Selpercatinib can influence its pharmacokinetics, resulting in alterations in bioavailability, metabolism, and elimination processes.
Which solvents help in the analysis of Selpercatinib impurities?
Methanol is used as a solvent in analytical techniques to separate and detect Selpercatinib impurities.
What steps can manufacturers take to control impurity levels in Selpercatinib?
Manufacturers can take various steps to control impurity levels in Selpercatinib, including using high-quality starting materials, optimizing reaction conditions, implementing effective purification techniques, and monitoring impurity levels throughout the manufacturing process.
What are the temperature conditions required to store Selpercatinib Impurities?
Selpercatinib Impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.