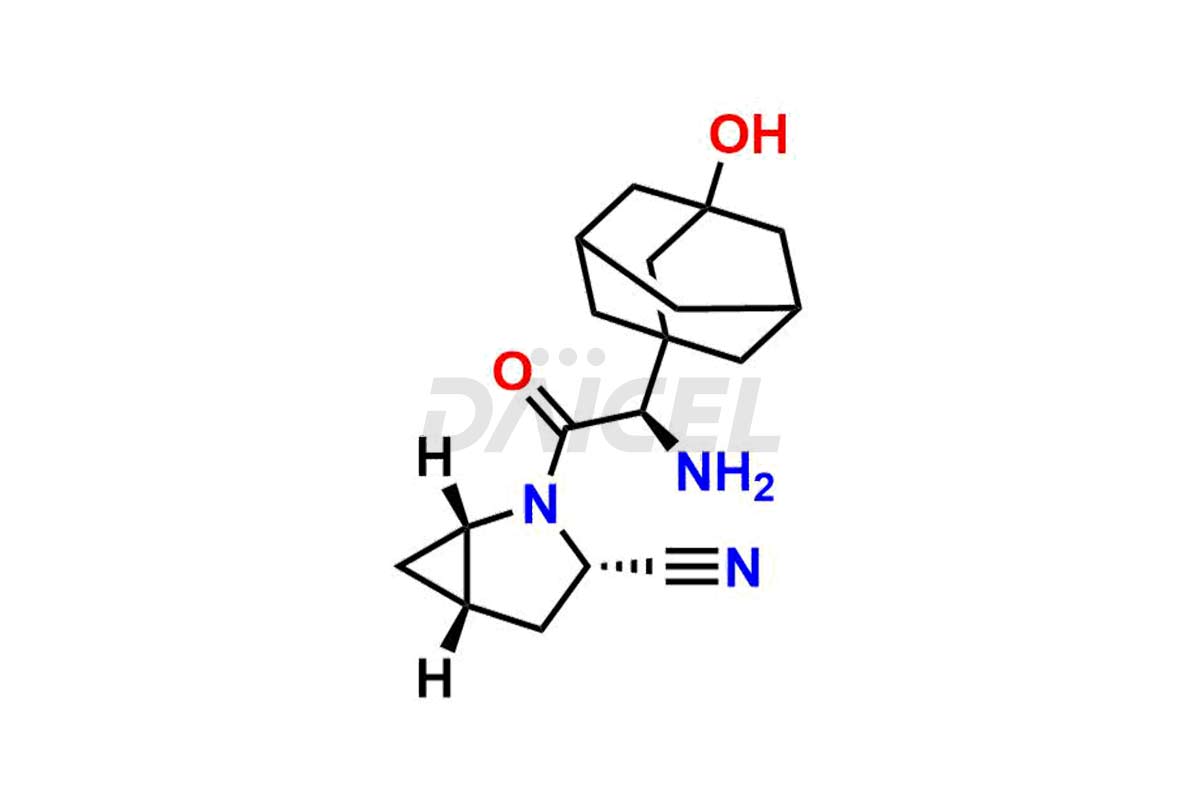
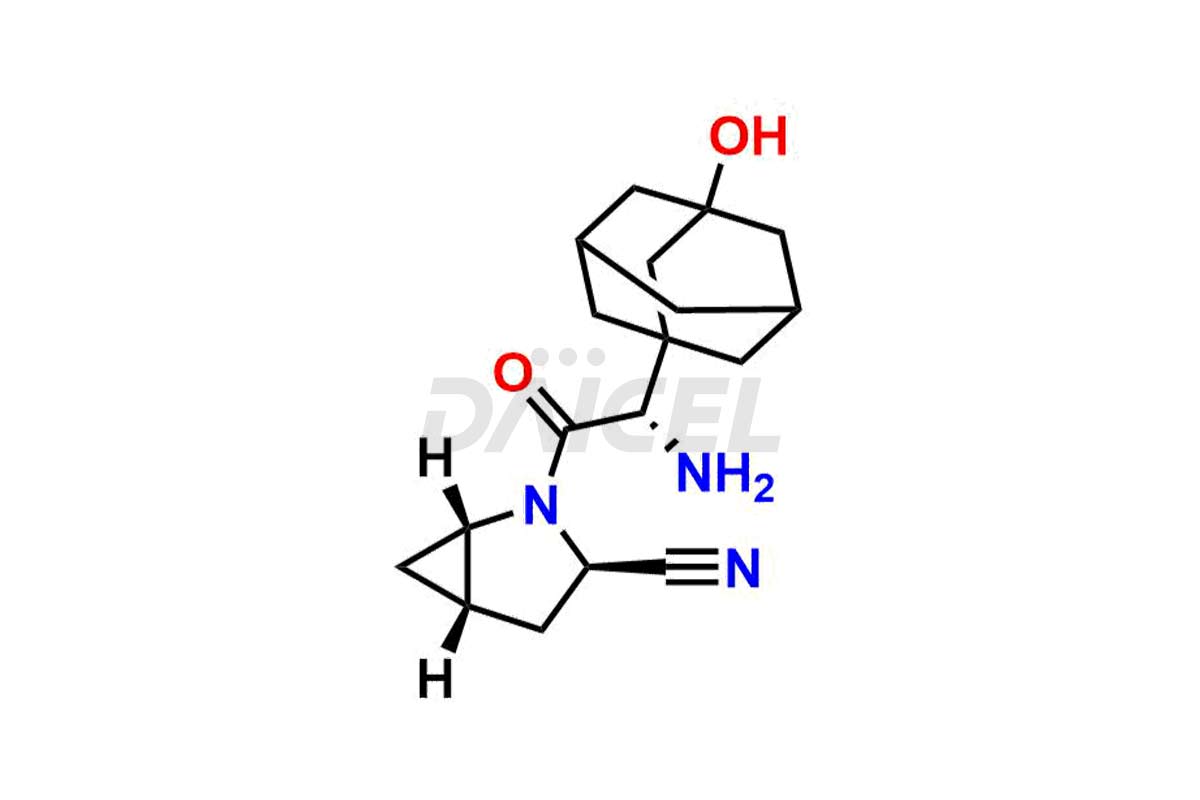
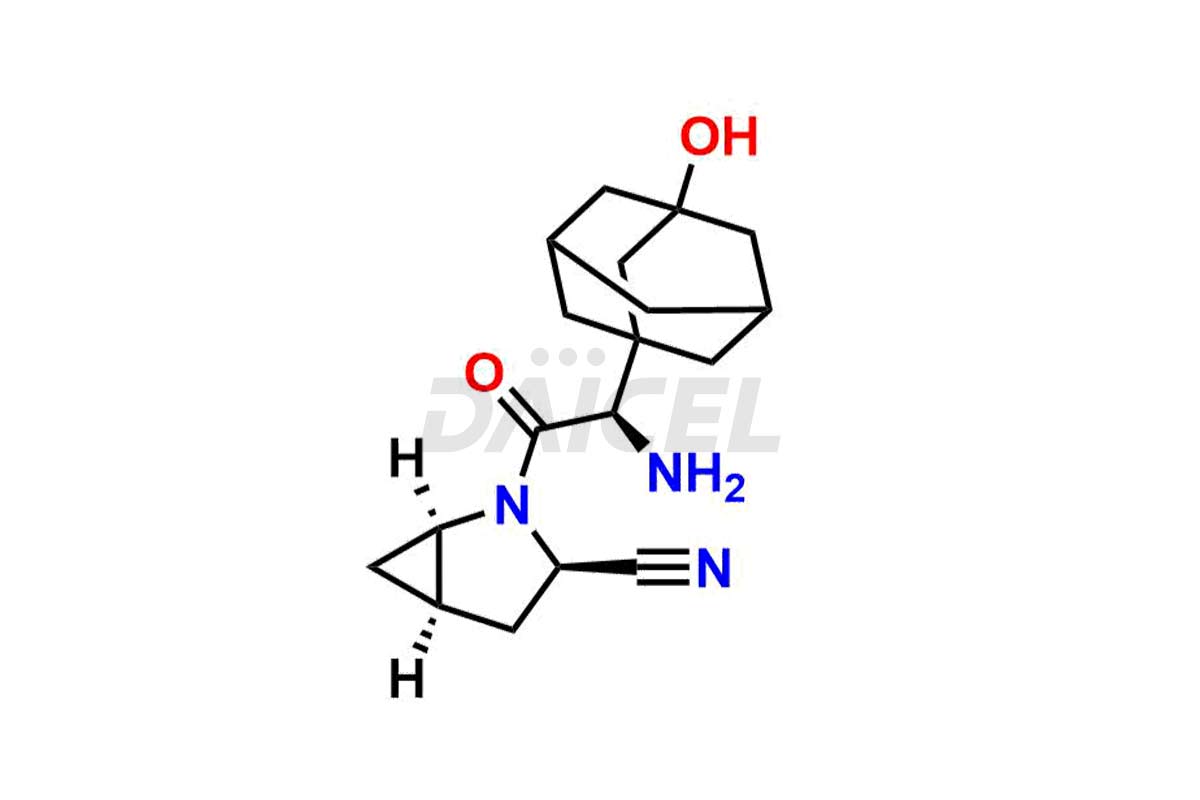
Saxagliptin
General Information
Saxagliptin Impurities and Saxagliptin
Daicel Pharma is a reliable source for synthesizing high-quality Saxagliptin impurities, specifically N-Boc-3,4-dihydroxy-(S)-adamantyl glycine, N-Boc-adamatyl glycine, Saxagliptin diastereoisomer-R, S, S, S, Saxagliptin diastereoisomer-S, R, S, S, Saxagliptin Imine Impurity, and Saxagliptin isomers-R, R, R, R. These impurities aid in evaluating Saxagliptin’s quality, stability, and safety. Daicel Pharma also provides a custom synthesis of Saxagliptin impurity standards that can be shipped anywhere worldwide to meet clients’ requirements.
Saxagliptin [CAS: 361442-04-8] is an oral antidiabetic medication belonging to the class of dipeptidyl peptidase-4 (DPP-4) inhibitors. It treats type 2 diabetes mellitus.
Saxagliptin: Use and Commercial Availability
Saxagliptin is an oral antihyperglycemic agent used to lower blood sugar levels in individuals with type 2 diabetes mellitus. It improves glycemic control with other diabetes medications such as metformin, sulfonylurea, or long- or intermediate-acting insulin (with or without metformin). Saxagliptin helps lower blood sugar levels by increasing the levels of GLP-1 and GIP. Saxagliptin is available under Onglyza.
Saxagliptin Structure and Mechanism of Action 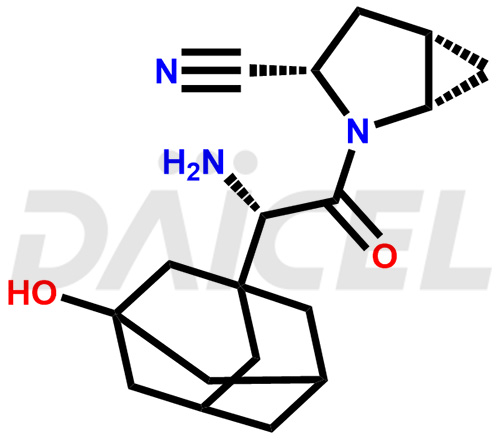
The chemical name of Saxagliptin is (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile. Its chemical formula is C18H25N3O2, and its molecular weight is approximately is 315.4 g/mol
Saxagliptin is a DPP4 inhibitor that inactivates incretin hormones. It reduces fasting and postprandial glucose concentrations in patients with type 2 diabetes mellitus.
Saxagliptin Impurities and Synthesis
Saxagliptin’s manufacturing process1 can develop impurities, which could diminish its effectiveness. They may come from various raw materials, intermediates, and chemicals used for Saxagliptin synthesis. It is necessary to manage and monitor these contaminants closely to ensure the drug’s effectiveness and safety.
Daicel Pharma offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Saxagliptin impurity standards such as N-Boc-3,4-dihydroxy-(S)-adamantyl glycine, N-Boc-adamatyl glycine, Saxagliptin diastereoisomer-R, S, S, S, Saxagliptin diastereoisomer-S, R, S, S, Saxagliptin Imine Impurity and Saxagliptin isomers-R, R, R, R, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT on request. Upon delivery, we give a complete characterization report. Daicel Pharma has the technology and expertise to prepare any unknown Saxagliptin impurities or degradation products.
References
FAQ's
References
- Robl, Jeffrey A.; Sulsky, Richard B.; Augeri, David J.; Magnin, David R.; Hamann, Lawrence G.; Betebenner, David A., Cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV and method, Bristol-Myers Squibb Co., United States, US6395767B2, May 28, 2002
- Xu, Xiaohui; Demers, Roger; Gu, Huidong; Christopher, Lisa J.; Su, Hong; Cojocaru, Laura; Boulton, David W.; Kirby, Mark; Stouffer, Bruce; Humphreys, William G.; et al, Liquid chromatography and tandem mass spectrometry method for the quantitative determination of saxagliptin and its major pharmacologically active 5-monohydroxy metabolite in human plasma: Method validation and overcoming specific and non-specific binding at low concentrations, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences Volume: 889-890, Pages: 77-86, 2012
Frequently Asked Questions
Why is the presence of impurities a concern in Saxagliptin?
Impurities in Saxagliptin can impact its quality, safety, and efficacy. Depending on the nature and level of impurities, the drug's pharmacological activity and stability may pose risks to the patient's health.
How are impurities detected and quantified in Saxagliptin?
Saxagliptin impurities are detected and quantified using analytical techniques such as high-performance liquid chromatography (HPLC) and the Ultraviolet method.
Which solvents help in the analysis of Saxagliptin impurities?
Water and Methanol are commonly used as a solvent in analytical techniques like high-performance liquid chromatography (HPLC) to separate and detect Saxagliptin impurities.
What are the temperature conditions required to store Saxagliptin Impurities?
Saxagliptin Impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.