Risperidone
General Information
Risperidone Impurities and Risperidone
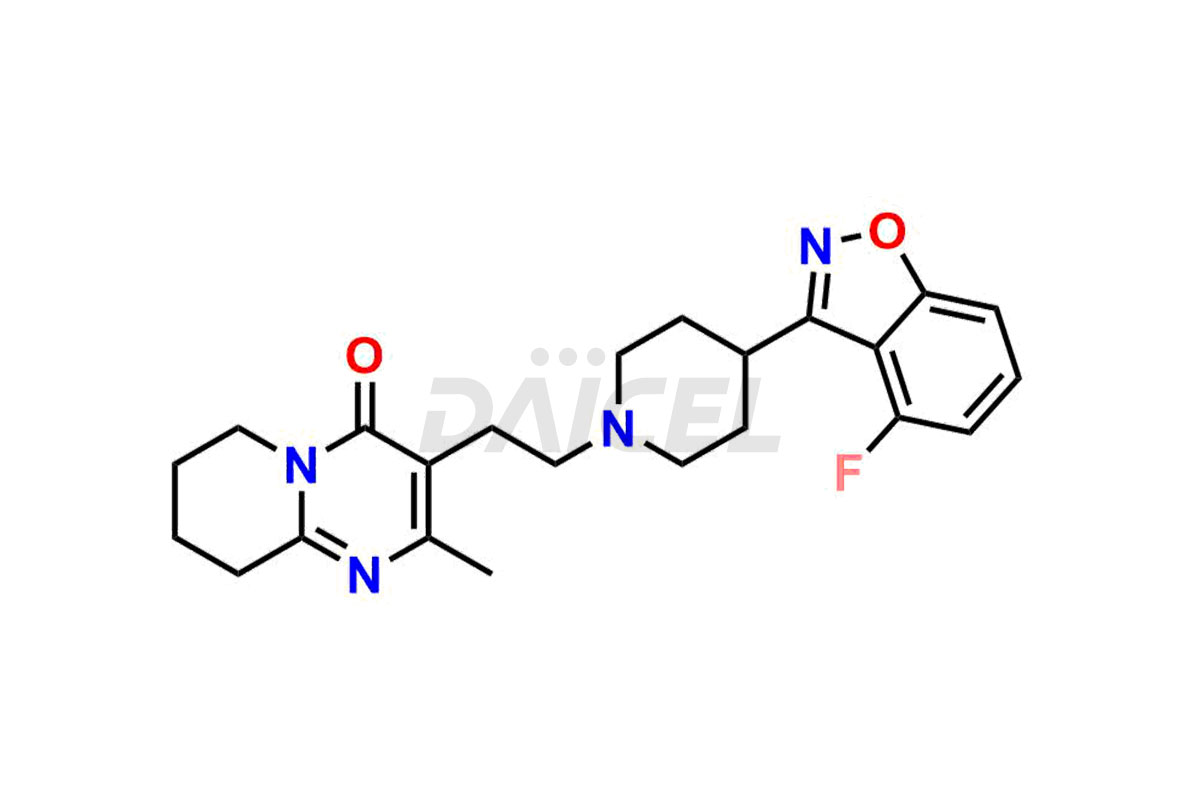
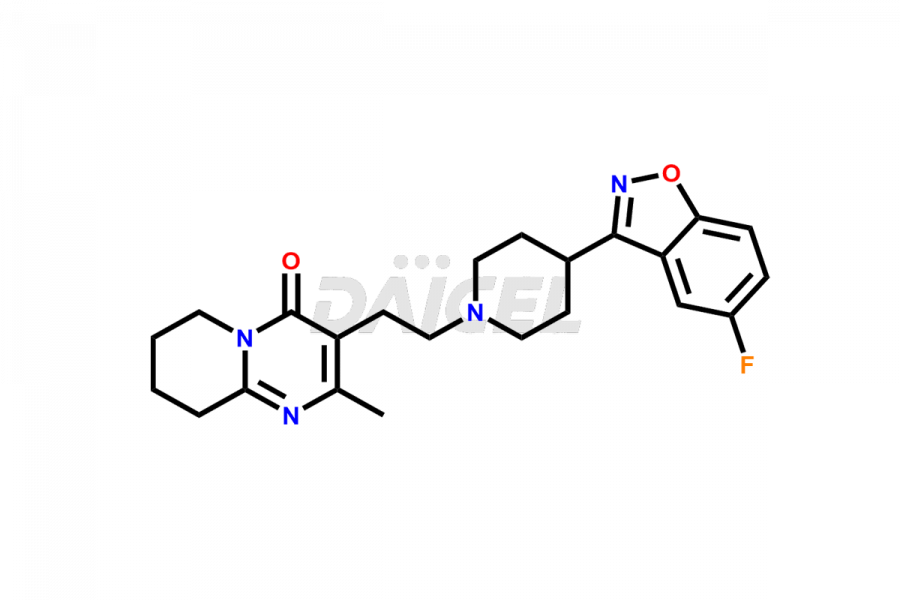
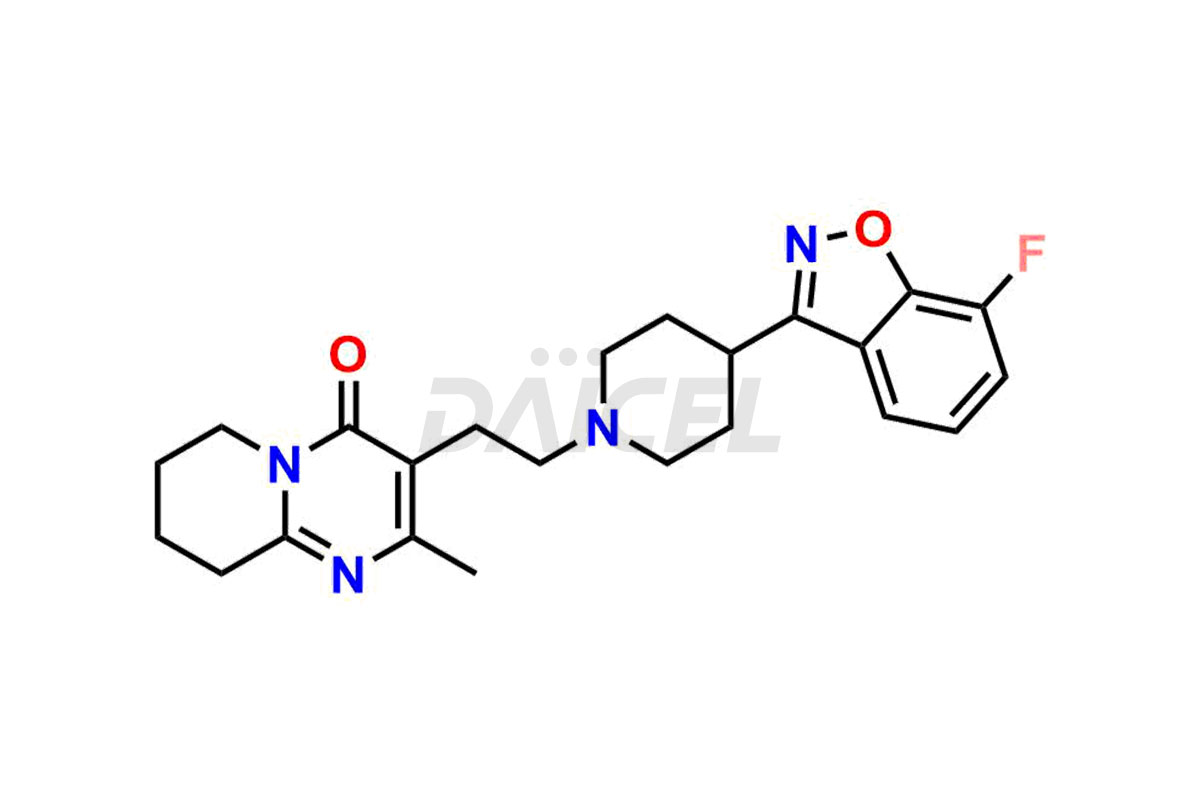
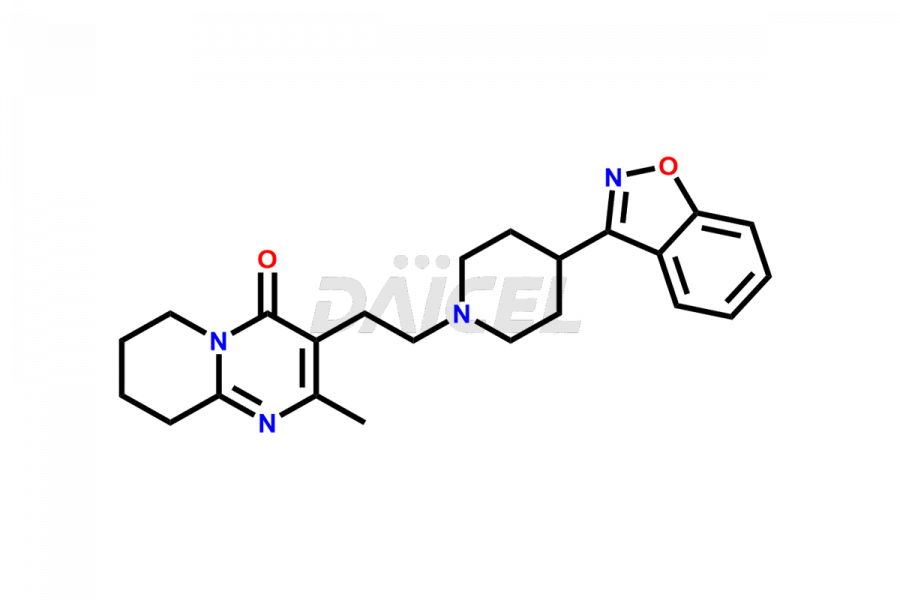
Daicel Pharma synthesizes Risperidone impurity standards, including Risperidone N-oxide, 7-Fluoro Risperidone, 4-Fluoro Risperidone, 5-Fluoro Risperidone, and Des Fluoro Risperidone. They are significant in assessing the quality, stability, and biological safety of Risperidone. Daicel Pharma offers custom synthesis for Risperidone impurities and delivers them globally.
Risperidone [CAS: 106266-06-2] belongs to the pyridopyrimidine class and a second-generation antipsychotic (SGA) medicine. Risperidone treats schizophrenia and bipolar disorder.
Risperidone: Use and Commercial Availability
Risperidone treats schizophrenia in adults and manic or mixed episodes of bipolar disorder in children and adolescents aged 10 to 17. It controls symptoms of inappropriate behavior due to aggression and or psychosis. This drug is available under Perseris Kit, Risperdal, Rykindo, and Uzedy.
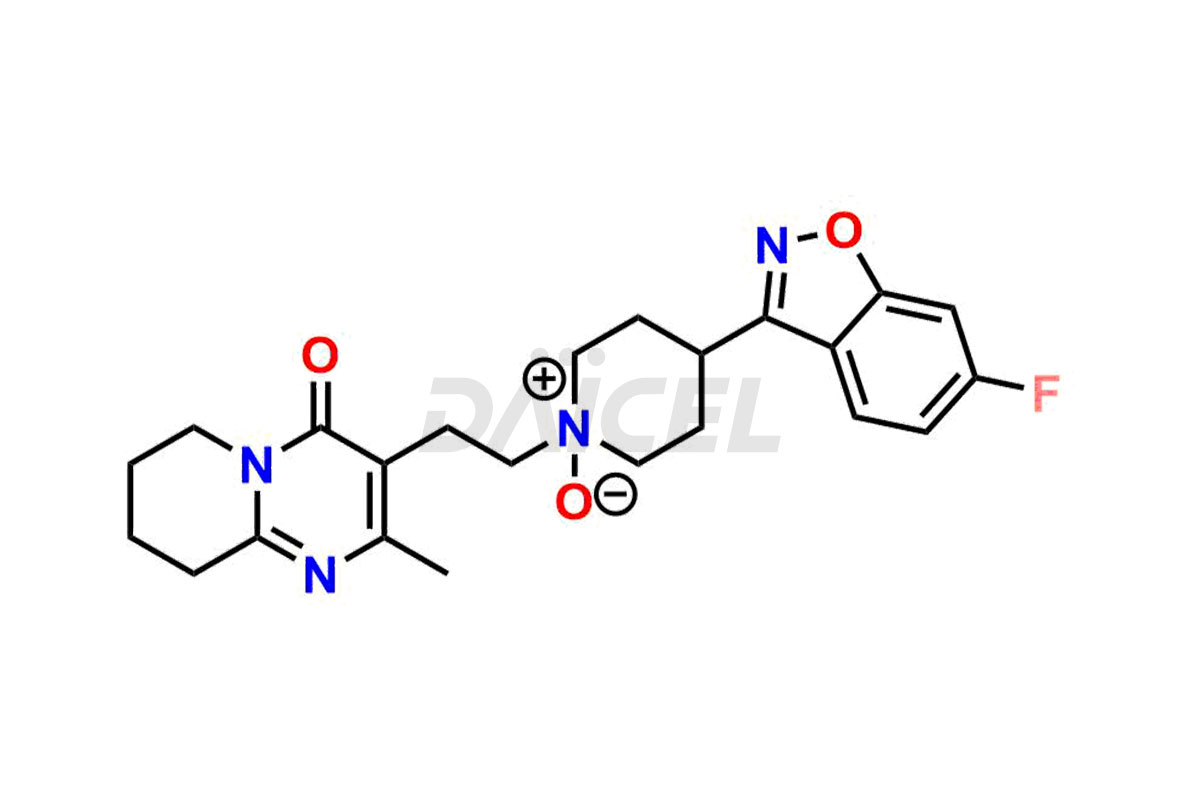
Risperidone Structure and Mechanism of Action 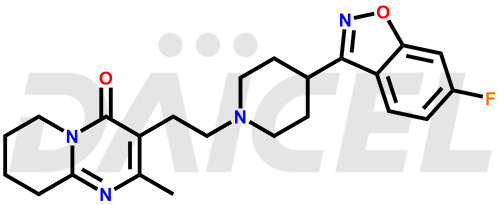
The chemical name of Risperidone is 3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one. Its chemical formula is C23H27FN4O2, and its molecular weight is approximately 410.5 g/mol.
The mechanism of action of Risperidone is unknown.
Risperidone Impurities and Synthesis
Risperidone, an atypical antipsychotic medicine, may include impurities that impair efficacy and safety. Their presence may be due to the synthetic process1 or storage conditions.
Daicel Pharma provides a Certificate of Analysis (CoA) of Risperidone impurity standards, including Risperidone N-oxide, 7-Fluoro Risperidone, 4-Fluoro Risperidone, 5-Fluoro Risperidone, and Des Fluoro Risperidone. Our CoA is from a cGMP-compliant analytical facility and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2,3. If requested, we give additional characterization data, such as 13C-DEPT. Daicel Pharma can provide unknown Risperidone impurities or degradation products.
References
FAQ's
References
- Kennis, Ludo Edmond Josephine; Vandenberk, Jan, 1,2-benzisoxazol-3-yl and 1,2-benzisothiazol-3-yl derivatives, Janssen Pharmaceutica N. V., Belgium, EP196132B1, August 12, 1992
- Woestenborghs, Robert; Lorreyne, Willy; Van Rompaey, Frank; Heykants, Jos, Determination of risperidone and 9-hydroxyrisperidone in plasma, urine and animal tissues by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 583, Issue: 2, Pages: 223-30, 1992
- Aravagiri, Manickam; Marder, Stephen R., Simultaneous determination of risperidone and 9-hydroxyrisperidone in plasma by liquid chromatography/electrospray tandem mass spectrometry, Journal of Mass Spectrometry, Volume: 35, Issue: 6, Pages: 718-724, 2000
Frequently Asked Questions
What is the detection process of Risperidone impurities?
Risperidone impurities are detected using analytical techniques such as high-performance liquid chromatography (HPLC).
How are Risperidone impurities monitored during synthesis?
Risperidone impurities are monitored during synthesis through rigorous quality control measures and analytical testing at various stages to ensure impurity levels remain within acceptable limits.
Can Risperidone impurities harm the effectiveness of the drug?
Risperidone impurities can potentially influence the effectiveness of the drug.
What are the temperature conditions required to store Risperidone impurities?
Risperidone impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.