Riociguat
General Information
Riociguat Impurities and Riociguat
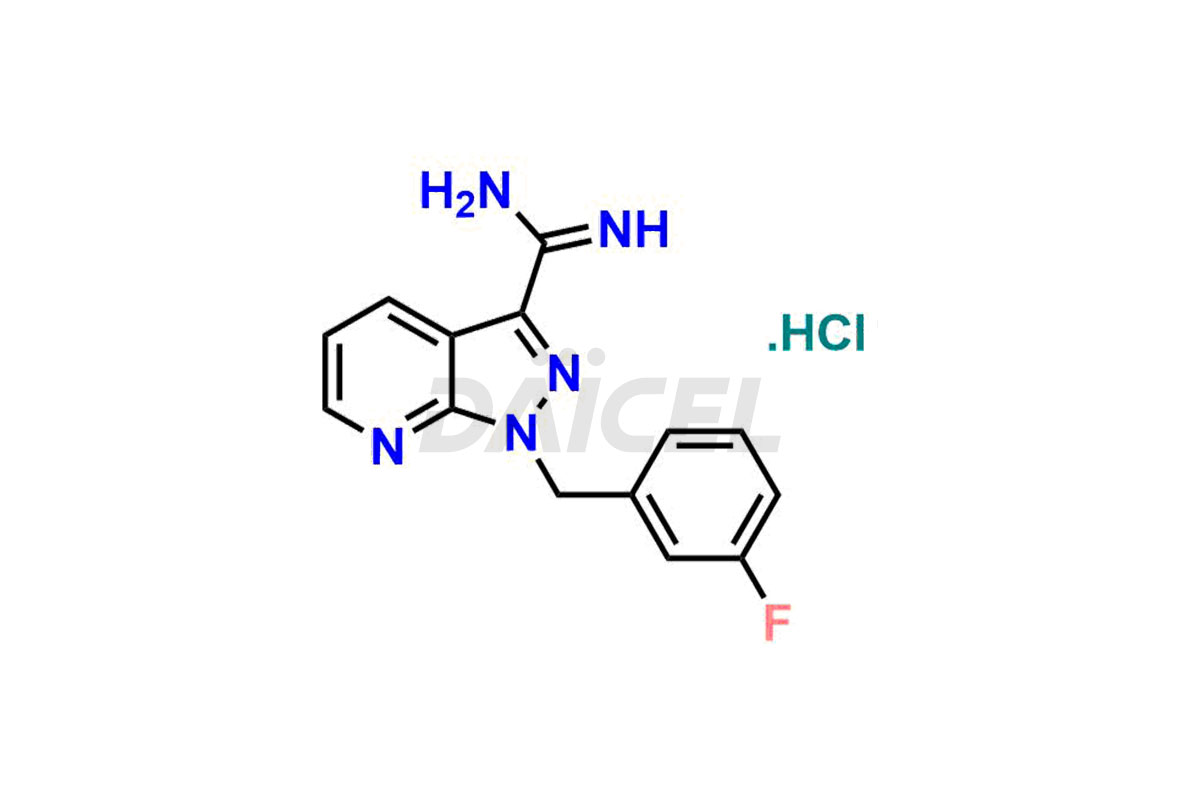
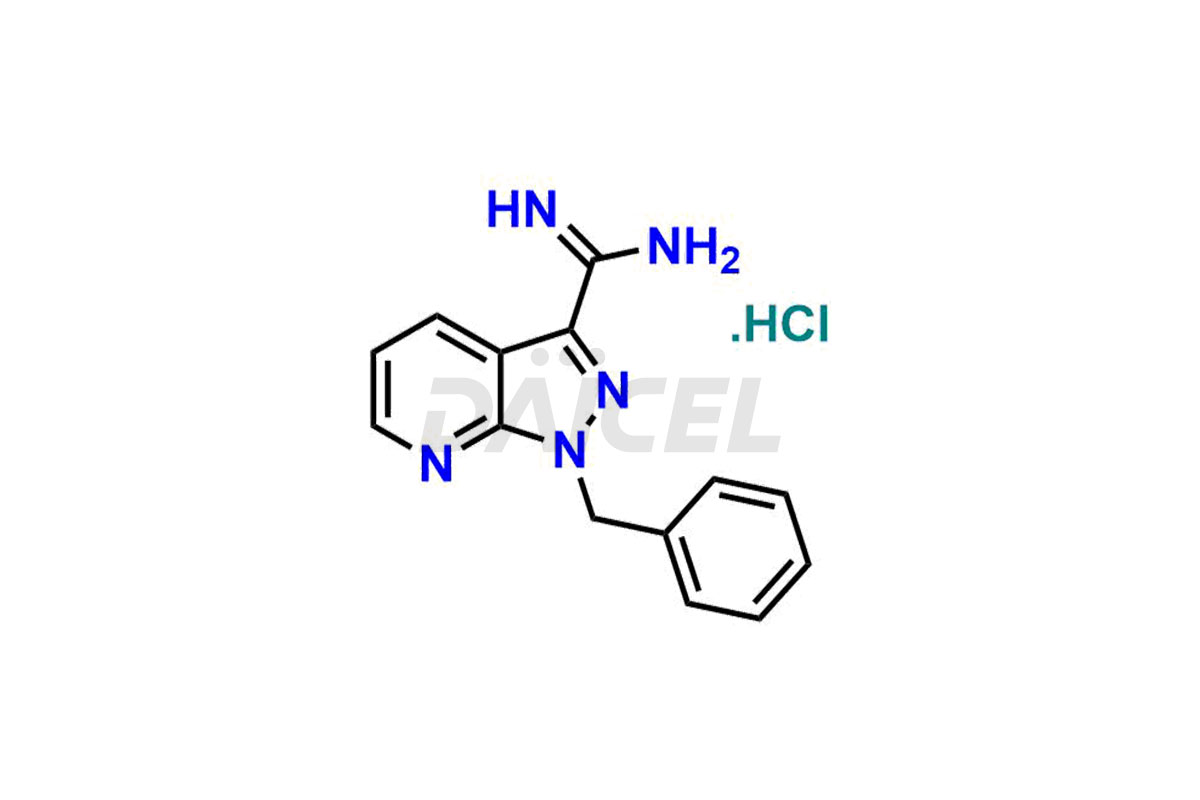
Daicel Pharma offer Riociguat impurity standards that include 1-(3-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [m-Fluro FPC], 1-(4-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [P-Fluro FPC], and 1-benzyl-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [Desfluro FPC]. They are vital to the quality, stability, safety, and efficient analysis of Riociguat. Daicel Pharma also provides custom synthesis for Riociguat and delivers it globally.
Riociguat [CAS: 625115-55-1] is an organofluorine compound and a carbamate ester. It acts as a soluble guanylate cyclase activator and an antihypertensive drug.
Riociguat: Use and Commercial Availability
Riociguat treats patients with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) following surgical treatment or inoperable CTEPH to enhance exercise capacity. This drug is available under the brand name Adempas.
Riociguat Structure and Mechanism of Action 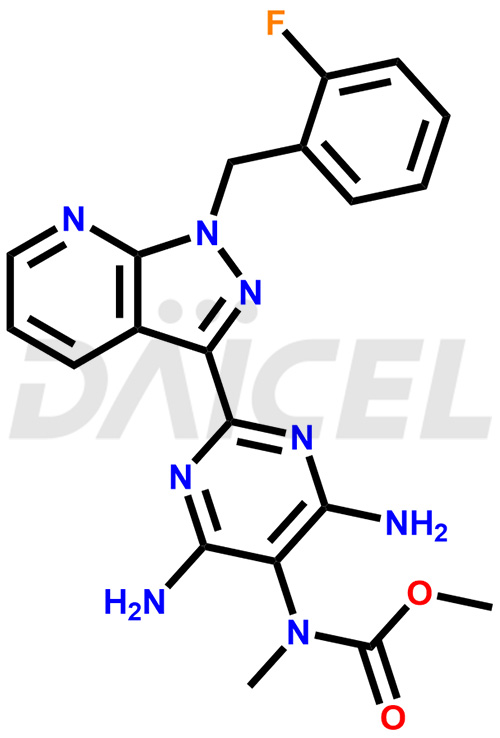
The chemical name of Riociguat is Methyl [4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]methylcarbamate. Its chemical formula is C20H19FN8O2, and its molecular weight is approximately 422.4 g/mol.
Riociguat stimulates an enzyme of the cardiopulmonary system, soluble guanylate cyclase (sGC). It increases the generation of cGMP and vasodilation.
Riociguat Impurities and Synthesis
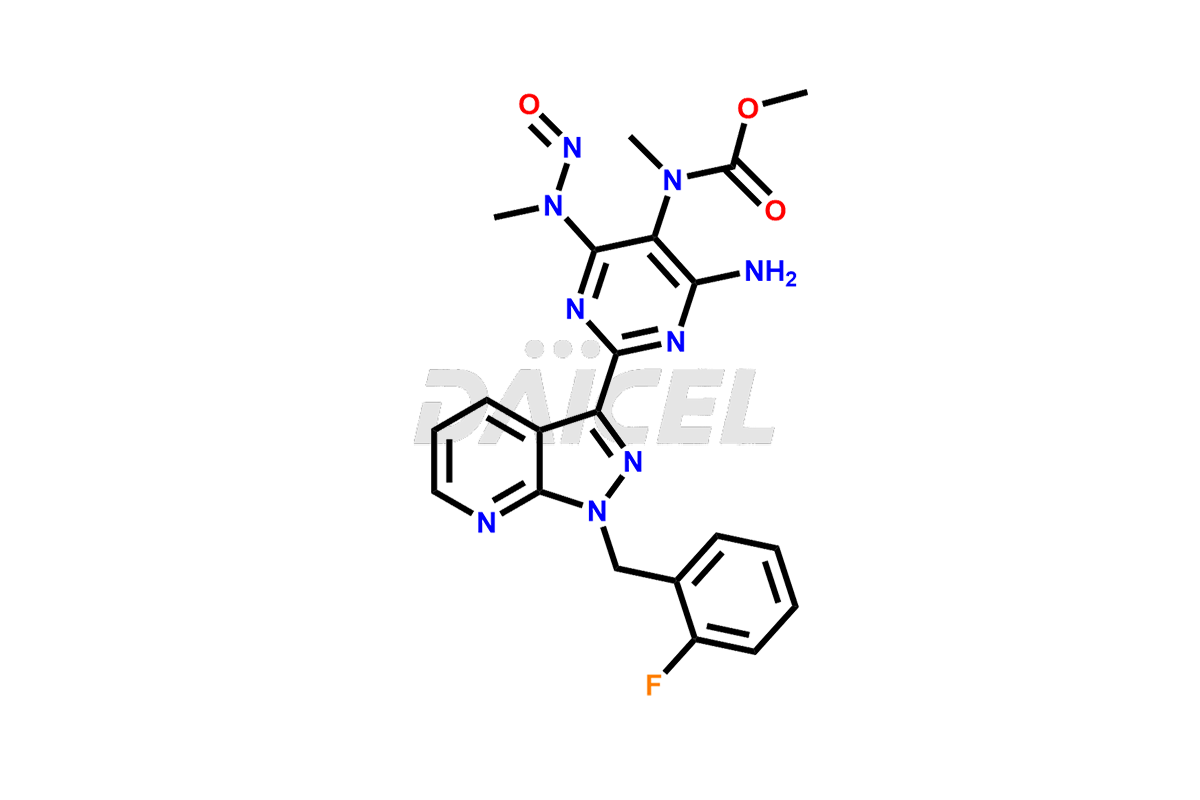
Riociguat may contain impurities that arise during manufacturing or from degradation. These impurities are closely monitored and controlled to ensure product quality and safety. Manufacturers employ purification methods and conduct rigorous analytical testing to minimize impurity levels. Regulatory authorities set limits for specific impurities in Riociguat to meet regulatory requirements.
Daicel Pharma offers a Certificate of Analysis (CoA) for Riociguat impurity standards that include 1-(3-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [m-Fluro FPC], 1-(4-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [P-Fluro FPC], and 1-benzyl-1H-pyrazolo[3,4-b]pyridine-3-carboximidamide hydrochloride [Desfluro FPC]. Daicel’s cGMP-certified analytical facility provides a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterizations like 13C-DEPT are available upon request. We offer Riociguat impurities and degradation products.
References
FAQ's
References
- Alonso-Alija, Cristina; Bischoff, Erwin; Muenter, Klaus; Stasch, Johannes-Peter; Stahl, Elke; Weigand, Stefan; Feurer, Achim, Carbamate-Substituted Pyrazolopyridines, Bayer Aktiengesellschaft, Germany, EP1506193B1, June 21, 2006
- Gorumutchu, Giri Prasad; Ratnakaram, Venkata Nadh; Malladi, Sireesha, Determination of riociguat by oxidative coupling using visible spectrophotometry, Oriental Journal of Chemistry, Volume: 35, Issue: Spec.Iss.1, Pages: 48-53, 2019
Frequently Asked Questions
Why is it vital to control Riociguat impurities?
Controlling Riociguat impurities is essential for drug safety, quality, and efficacy.
How are Riociguat impurities characterized?
Various analytical techniques identify and characterize impurities. Impurities are properly quantified using reference standards and verified analytical procedures.
Can Riociguat impurities be minimized?
Various strategies are applied to control and minimize impurities during synthesis. It includes employing high-quality starting materials, following proper purification procedures, and performing quality control testing.
What are the temperature conditions required to store Riociguat impurities?
Riociguat impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.