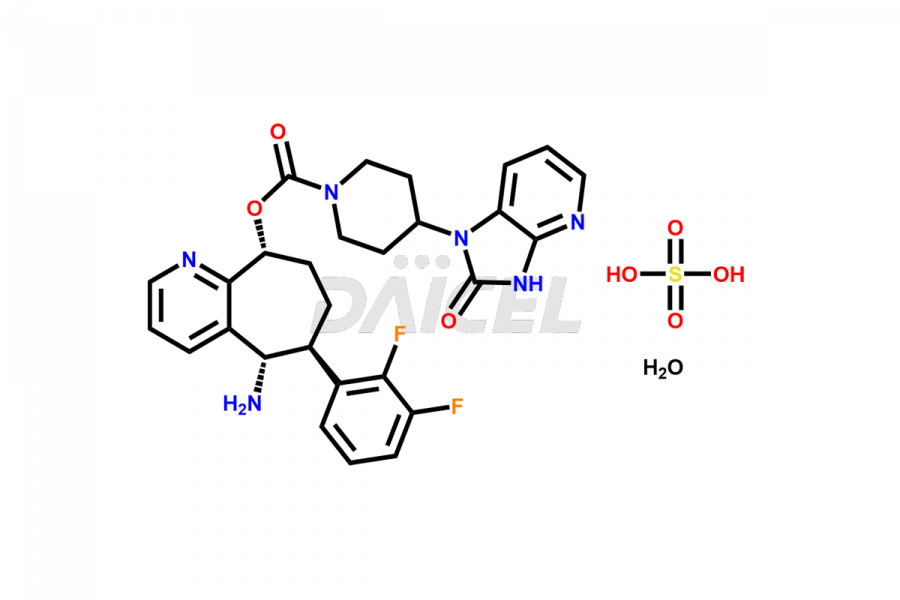
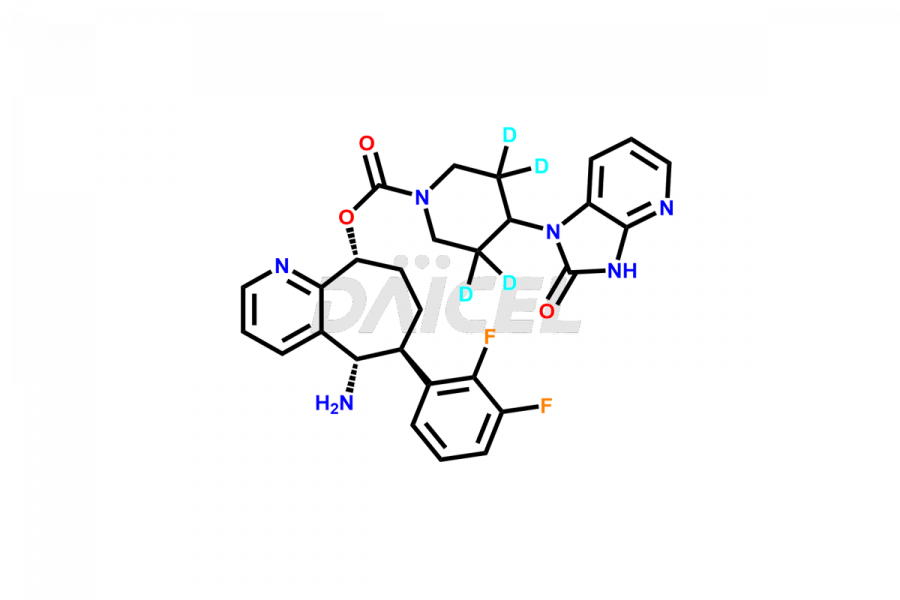
Rimegepant
General Information
Rimegepant Impurities and Rimegepant
Daicel Pharma is capable of synthesizing Rimegepant impurity standard, Rimegepant Sulfate and more. This impurity is essential for the quality, stability, safety, and efficiency analysis of Rimegepant. Furthermore, Daicel Pharma offers custom synthesis for Rimegepant synthesis for global delivery.
Rimegepant [CAS: 1289023-67-1] is an oral calcitonin gene-related peptide (CGRP) receptor antagonist for treating migraine headaches.
Rimegepant: Use and Commercial Availability
Rimegepant treats and prevents migraine with or without aura in patients. It prevents episodic migraine attacks in adults. This drug is available under the brand name Nurtec ODT.
Rimegepant Structure and Mechanism of Action 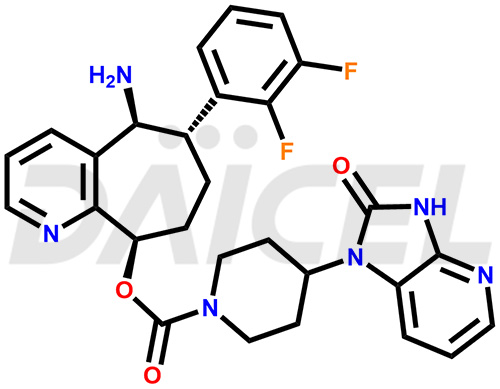
The chemical name of Rimegepant is 4-(2,3-dihydro-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl)- 1-Piperidinecarboxylic acid (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-yl ester. Its chemical formula is C28H28F2N6O3, and its molecular weight is approximately 534.6 g/mol.
Rimegepant prevents calcitonin gene-related peptide (CGRP) actions and treats migraine headache pain.
Rimegepant Impurities and Synthesis
Rimegepant, a medication for acute migraines, may contain impurities that arise during manufacturing1 or from degradation. These impurities are closely monitored and controlled to ensure product quality and safety. Manufacturers employ purification methods and conduct rigorous analytical testing to minimize impurity levels.
Daicel Pharma offers a Certificate of Analysis (CoA) for Rimegepant impurity standard, Rimegepant Sulfate. Our cGMP-certified analytical laboratory provides this CoA and includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, are provided on request. Daicel Pharma offers Rimegepant impurities and labeled compounds for testing the efficacy of generic Rimegepant. We can provide Rimegepant-D4, a deuterium-labeled Rimegepant standard, for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Luo, Guanglin; Dubowchik, Gene M.; Macor, John E., CGRP receptor antagonists, Bristol-Myers Squibb Company, United States, US8314117B2, November 20, 2012
- Zheng, Naiyu; Zeng, Jianing; Akinsanya, Billy; Buzescu, Adela; Xia, Yuan-Qing; Ly, Van; Trouba, Kevin; Peng, Qianping; Aubry, Anne-Francoise; Arnold, Mark E., A rapid, accurate and robust UHPLC-MS/MS method for quantitative determination of BMS-927711, a CGRP receptor antagonist, in plasma in support of non-clinical toxicokinetic studies, Journal of Pharmaceutical and Biomedical Analysis Volume: 83, Pages: 237-248, 2013
Frequently Asked Questions
Can Rimegepant impurities be removed?
Depending on the impurity and its concentration, purification techniques and methods can be employed to remove or reduce them in Rimegepant.
Are there regulatory guidelines for Rimegepant impurities?
Regulatory authorities provide guidelines and limits for the level of impurities in pharmaceutical substances, including Rimegepant, to ensure product quality and safety.
What are the temperature conditions to control Rimegepant impurities?
The temperature conditions to control Rimegepant impurities typically involve storage and handling at specific temperature ranges, as specified in the drug's stability data and regulatory guidelines.
What are the temperature conditions required to store Rimegepant impurities?
Rimegepant impurities should be stored at a controlled room temperature between 2-8°C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.