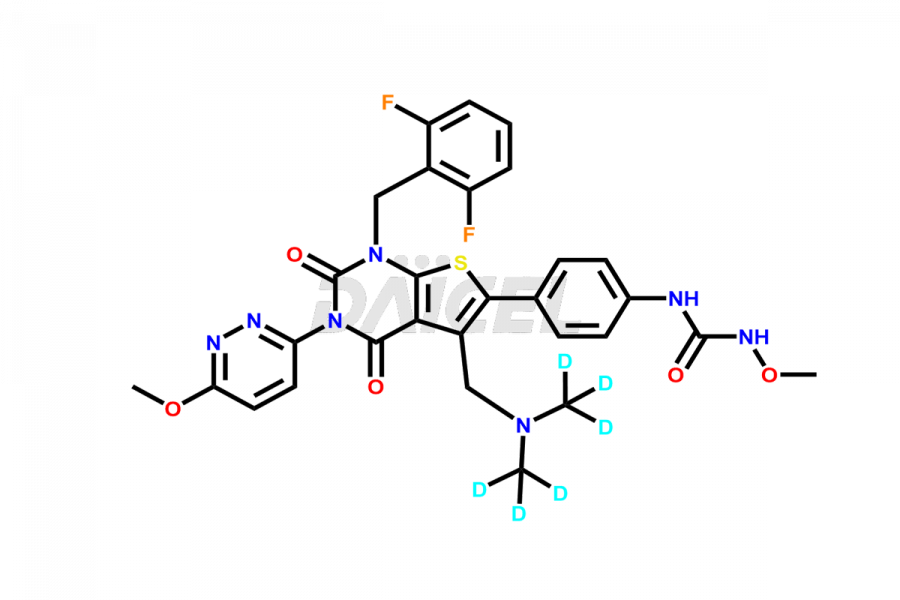
Relugolix
References
- Cho, Nobuo; Imada, Takashi; Hitaka, Takenori; Miwa, Kazuhiro; Kusaka, Masami; Suzuki, Nobuhiro, “Preparation of thienopyrimidine derivatives as gonadotropin-releasing hormone antagonists”, Takeda Chemical Industries, Ltd., US7300935B2, Nov 27, 2007
- Zhou, Bugao; Shen, Yuqi; Xu, Guanghui; Hui, Jian; Zhang, Mingyu, “Preparation of relugolix impurity pyridazine derivative” Nanjing F&S Pharmatech Co., Ltd., Jiangsu Litaier Pharmaceutical Co., Ltd., China, CN114478503A, May 13, 2022
Frequently Asked Questions
What types of Relugolix impurities may be present in the drug?
The most common types of Relugolix impurities are related compounds, residual solvents, and heavy metals.
What are the common degradation impurities of Relugolix?
The common Relugolix degradation impurities include acid impurities, oxidative impurities and other impurities.
How are Relugolix impurities detected and quantified?
Impurities in Relugolix are detected and quantified using various analytical techniques such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
How to remove Relugolix impurities during the synthesis of the drug?
During the manufacturing process of Relugolix, various purification techniques such as recrystallization and chromatography help to remove impurities.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.