Rasagiline
General Information
Rasagiline Impurities and Rasagiline
Daicel Pharma offers a Rasagiline impurity standard, N-Nitroso Rasagiline and more. This impurity is valuable for Rasagiline efficiency, stability, safety, and quality assessment. Daicel Pharma can synthesize Rasagiline impurities and can deliver them internationally.
Rasagiline [CAS: 136236-51-6] is an indane derivative that treats idiopathic Parkinson’s disease. It is a propargylamine-based drug.
Rasagiline: Use and Commercial Availability
Rasagiline is used as an initial monotherapy and as an adjunct therapy to levodopa to treat the signs and symptoms of idiopathic Parkinson’s disease. This medication is available in the market under the tradename of Azilect.
Rasagiline Structure and Mechanism of Action 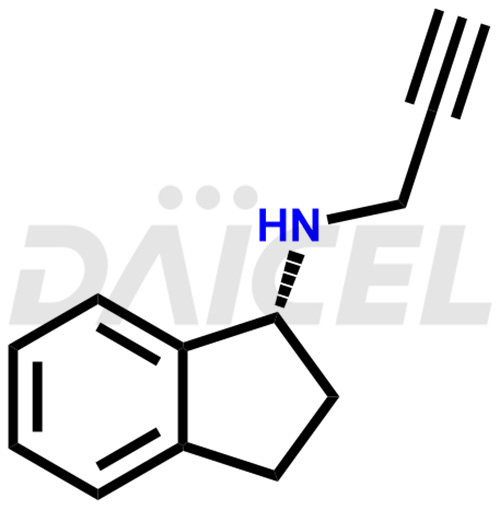
The chemical name of Rasagiline is (1R)-2,3-Dihydro-N-2-propyn-1-yl-1H-inden-1-amine. Its chemical formula is C12H13N, and its molecular weight is approximately 171.24 g/mol.
Rasagiline irreversibly blocks monoamine oxidase. Its mechanism of action is unknown.
Rasagiline Impurities and Synthesis
Rasagiline impurities refer to unintended chemical compounds present during the synthesis1 storage of Rasagiline, a medication treating patients with Parkinson’s disease. These impurities can arise from various sources, including starting materials, reagents, intermediates, or degradation products. Rasagiline impurities require strict control and monitoring to assure drug safety, efficacy, and quality, and analytical procedures to identify, quantify, and characterize these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for the Rasagiline impurity standard which is N-Nitroso Rasagiline. Our CoA is from our cGMP-certified analytical laboratory and includes detailed characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, can be provided on request. Daicel Pharma specializes in synthesizing Rasagiline impurities and labeled compounds. We provide N-Nitroso Rasagiline-D4, a deuterium-labeled Rasagiline compound, essential for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Youdim, Moussa B. H.; Finberg, John P. M.; Levy, Ruth; Sterling, Jeffrey; Lerner, David; Berger-Paskin, Tirtsah, R-Enantiomer of N-propargyl-1-aminoindan, its preparation and pharmaceutical compositions containing it, Teva Pharmaceutical Industries Ltd., Israel and Technion Research and Development Foundation Ltd., Israel, EP436492B1, June 8, 1994
- Ma, Jinfei; Chen, Xiaoyan; Duan, Xiaotao; Deng, Pan; Wang, Hui; Zhong, Dafang, Validated LC-MS/MS method for quantitative determination of rasagiline in human plasma and its application to a pharmacokinetic study, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 873, Issue: 2, Pages: 203-208, 2008
Frequently Asked Questions
Can Rasagiline impurities affect the effectiveness of the drug?
Yes, Rasagiline impurities can affect the drug's effectiveness as they can alter its pharmacological properties and lead to variations in therapeutic response.
Do regulatory authorities regulate Rasagiline impurities?
Yes, Rasagiline impurities are regulated by authorities to ensure the safety and quality of the medication.
What is the significance of controlling Rasagiline impurities?
The significance of controlling Rasagiline impurities is to ensure the safety, efficacy, and quality of the medication, as impurities can potentially affect its therapeutic effectiveness and may pose health risks to patients.
What are the temperature conditions required to store Rasagiline impurities?
Rasagiline impurities should be stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.



