LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma synthesizes more than ten high-quality Ranolazine impurities, 4-Chloro Ranolazine Impurity, 4-Methyl Ranolazine Impurity, 3,4-Dimethyl Ranolazine Impurity, Ranolazine ether dimer, Ranolazine Related compound-B and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Ranolazine. Moreover, Daicel Pharma offers custom synthesis of Ranolazine impurities and delivers them globally.
Ranolazine [CAS: 95635-55-5] was US-FDA approved in 2006 for treating chronic angina. It is classified as an anti-arrhythmic drug and is a piperazine derivative. Ranolazine effectively treats chronic angina without affecting heart rate or blood pressure, which sets it apart from other antianginal agents.
Ranolazine is a medicine prescribed to alleviate the symptoms of stable angina pectoris, characterized by chest pain caused by reduced blood flow to the heart. It is used as an add-on with other medicines, such as beta-blockers or calcium antagonists, when these medicines alone are not sufficient to control the disease, or when they cannot be taken by the patient. The drug is available under the brand names Ranexa and Aspruzyo Sprinkle.
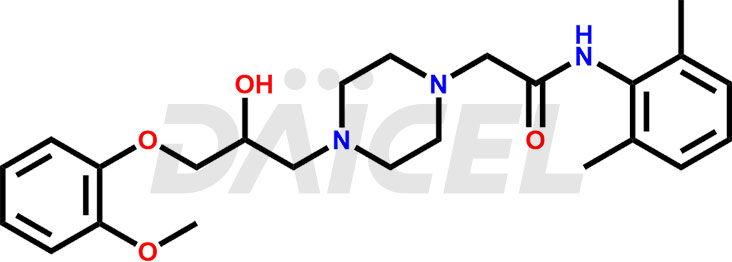
The chemical name of Ranolazine is N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]- 1-Piperazineacetamide. Its chemical formula is C24H33N3O4, and its molecular weight is approximately 427.5 g/mol.
Ranolazine has an antianginal effect, but its mechanism of action is unknown.
The impurities formed in Ranolazine API1 include process-related substances, degradation products, and residual solvents. It is essential to monitor and control the levels of these impurities during the manufacturing process to ensure the quality and safety of the finished drug product.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for more than ten Ranolazine impurity standards, 4-Chloro Ranolazine Impurity, 4-Methyl Ranolazine Impurity, 3,4-Dimethyl Ranolazine Impurity, Ranolazine ether dimer, Ranolazine Related compound-B and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Ranolazine impurity or degradation product. We also provide labeled compounds to quantify the efficacy of Ranolazine. Daicel offers highly pure Ranolazine-D3 and Ranolazine-D8, which are deuterium-labeled standards of Ranolazine for bioanalytical research and BA/BE studies with isotope data in CoA.
Methanol is the solvent for analyzing many Ranolazine impurities.
Ranolazine impurities should be stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Ranolazine impurities can be eliminated or removed through appropriate purification steps such as chromatography, crystallization, or recrystallization. Additionally, degradation impurities can be removed by following cGMP practices.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.