LOAD MORE
You're viewed 9 of 12 products
Daicel Pharma offers Rabeprazole impurity standards which include Rabeprazole Sulfone, Rabeprazole Impurity-5, Rabeprazole Impurity-2, Rabeprazole Sulfone N-Oxide, Rabeprazole Related compound-F, Rabeprazole Related Compound A and more. Their presence can influence the effectiveness, stability, and safety of Rabeprazole. Daicel Pharma offers Rabeprazole impurities with international delivery convenience to meet our client’s needs.
Rabeprazole [CAS: 117976-89-3] belongs to the benzimidazole, sulfoxide, and pyridine groups. It is an anti-ulcer drug and treats stomach acid production. It is a proton pump inhibitor.
Rabeprazole is used to treat acid reflux disease (GERD), peptic ulcer disease, H. pylori eradication, and prevent gastrointestinal bleeding when combined with NSAIDs. This drug is marketed under the tradenames of Aciphex and Aciphex Sprinkle.
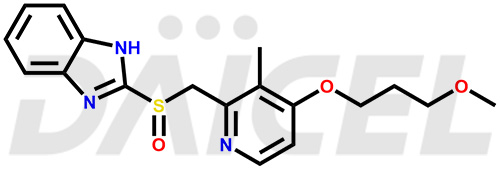
The chemical name of Rabeprazole is 2-[[[4-(3-Methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole. Its chemical formula is C18H21N3O3S, and its molecular weight is approximately 359.4 g/mol.
Rabeprazole blocks gastric H+, K+ ATPase at the secretory surface of gastric cells.
Rabeprazole manufacture and storage can result in the formation of impurities, which can affect its purity and efficacy. It is critical to verify the purity of the starting components and precisely manage the reaction conditions during the synthetic process1 to reduce impurity formation.
Daicel Pharma provides a Certificate of Analysis (CoA) for Rabeprazole impurity standards such as Rabeprazole Sulfone, Rabeprazole Impurity-5, Rabeprazole Impurity-2, Rabeprazole Sulfone N-Oxide, Rabeprazole Related compound-F, Rabeprazole Related Compound A, and more. The Certificate of Analysis (CoA) contains a detailed characterization report with information from techniques, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon request, we can supply supplementary data such as 13C-DEPT. Further, upon request, Daicel Pharma can offer unknown Rabeprazole impurities.
Regulatory authorities play a crucial role in monitoring and setting standards for Rabeprazole impurities to ensure the safety and efficacy of the drug.
Yes, Rabeprazole impurities are evaluated during clinical trials to assess their safety and ensure compliance with regulatory standards.
Yes, impurities in Rabeprazole can impact the drug's stability.
Rabeprazole impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.