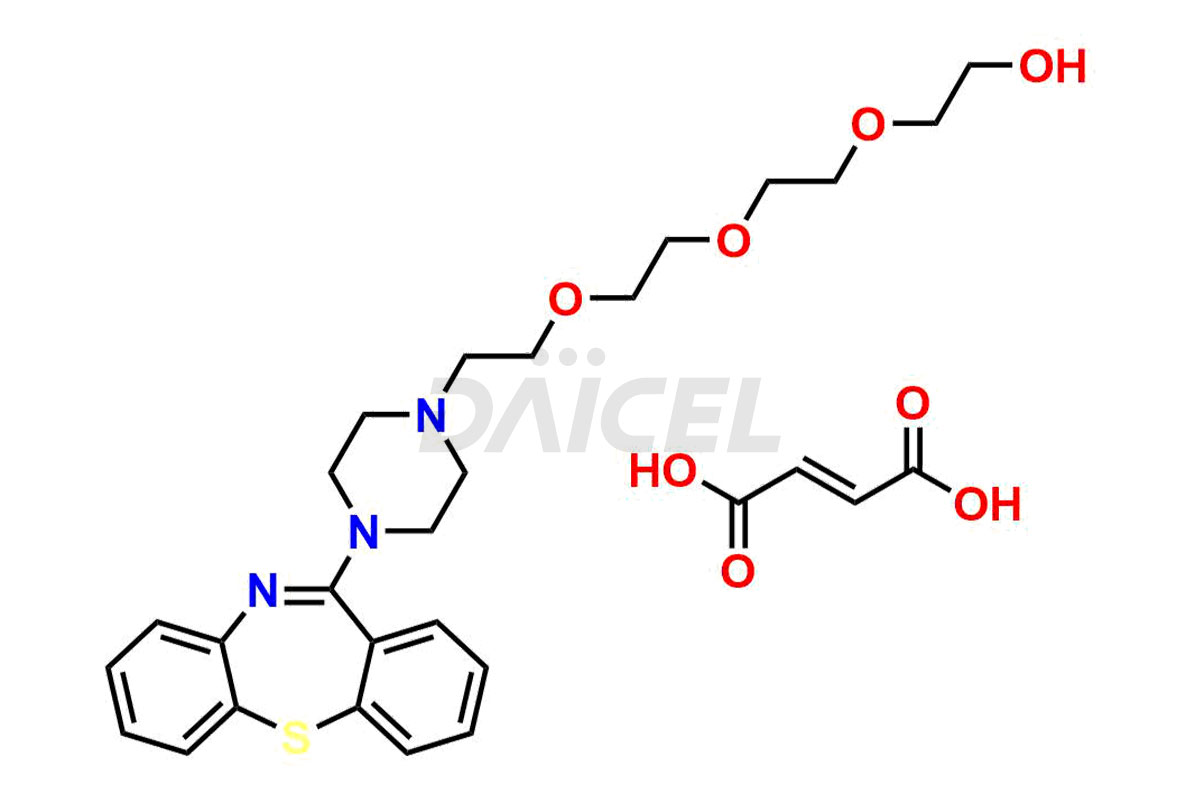
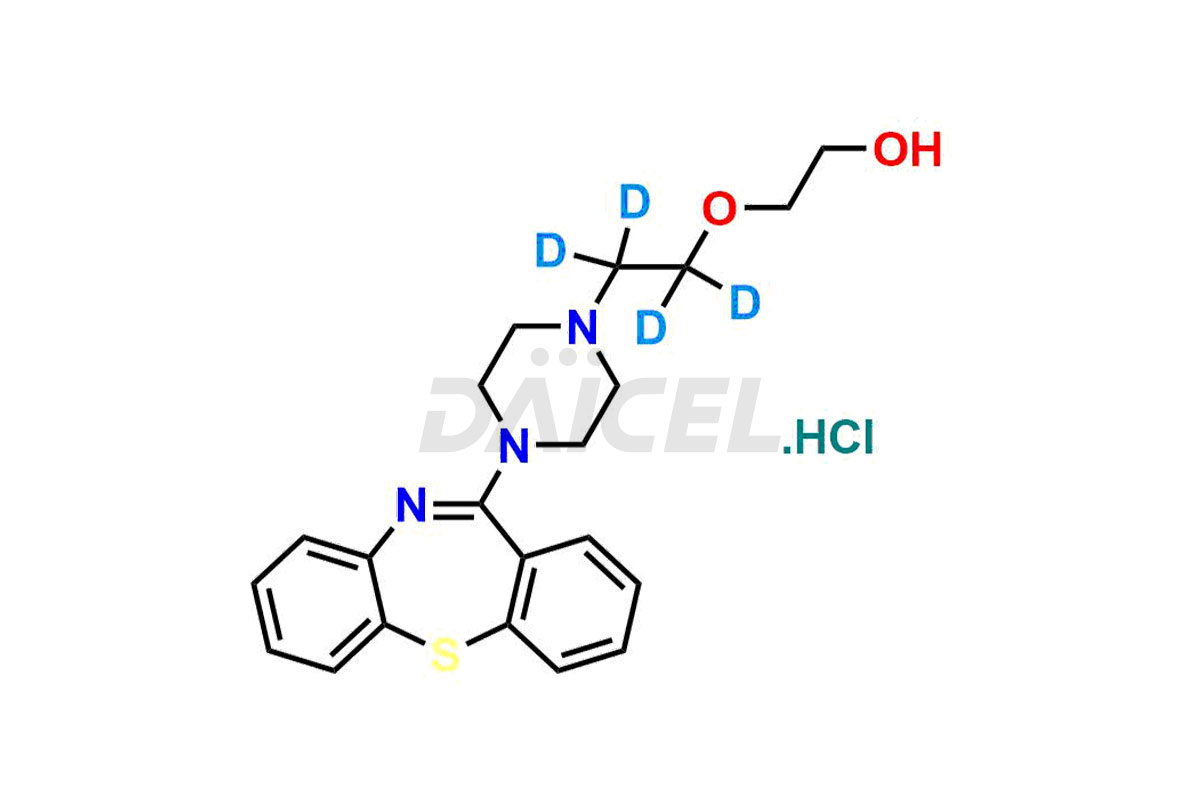
Quetiapine
General Information
Quetiapine Impurities and Quetiapine
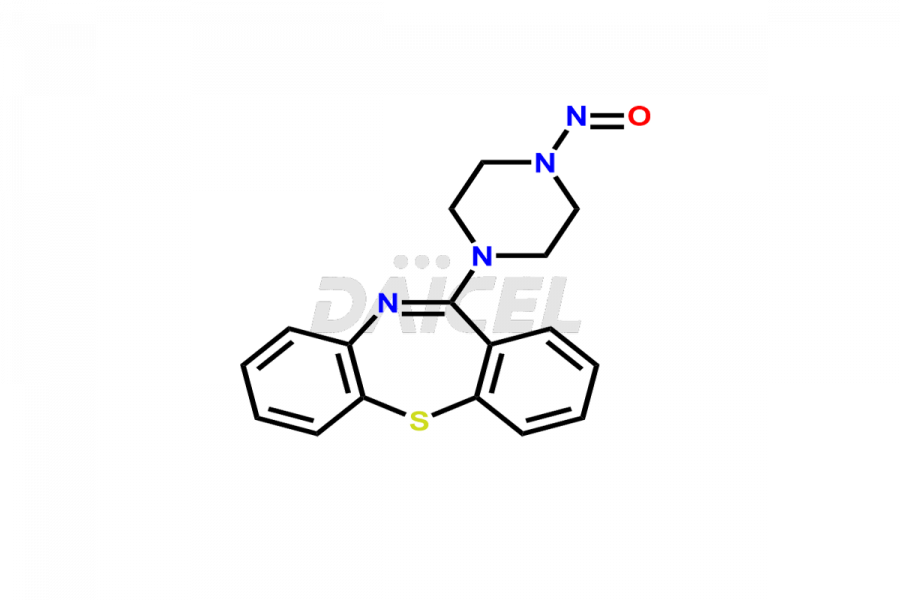
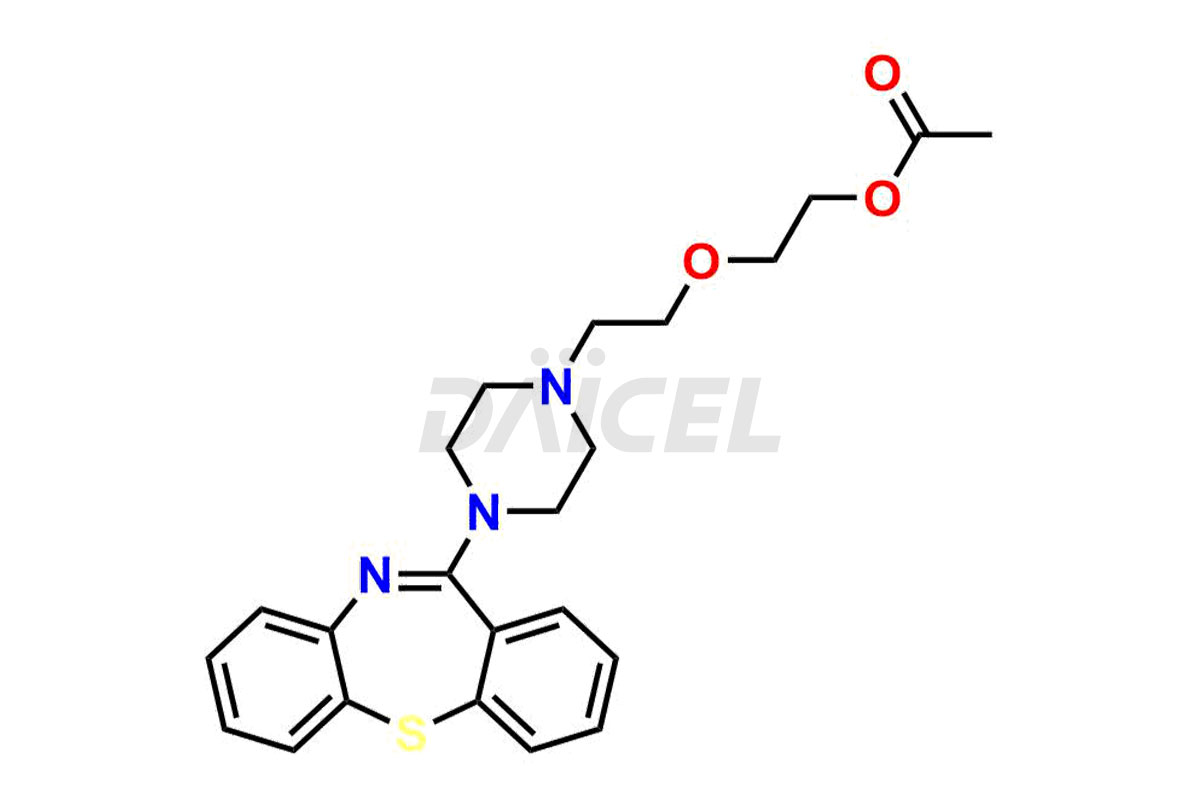
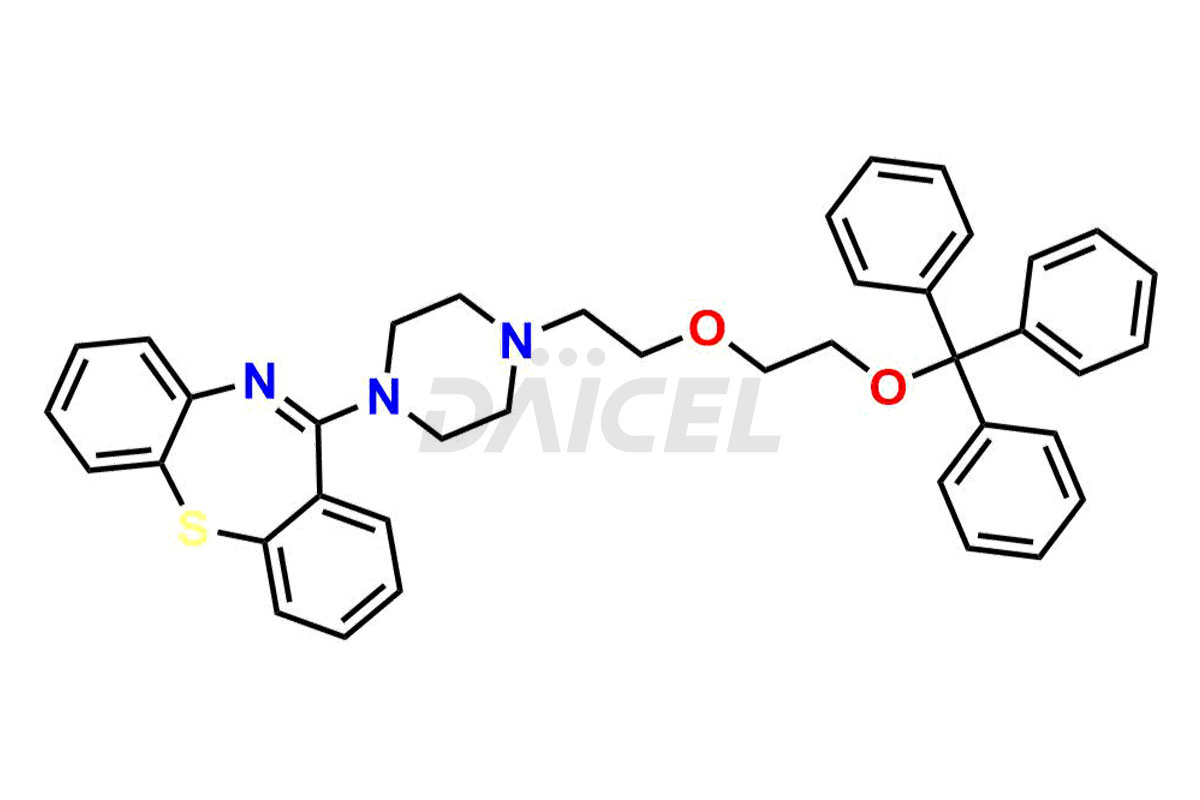
Daicel Pharma offers high-quality Quetiapine impurity standards, including standards Quetiapine Imp-A, Quetiapine Imp-O, Quetiapine Tetra Ethylene Glycol Fumarate Salt, Nitroso aryl piperazine, and 11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine. Their presence can impact the effectiveness, stability, and safety of Quetiapine. Daicel Pharma provides custom Quetiapine impurities, ensuring worldwide delivery to cater to the unique requirements of our clients.
Quetiapine [CAS: 111974-69-7] is a N-alkylpiperazine, a dibenzothiazepine, and a N-arylpiperazine. Quetiapine is for treating various mental health conditions.
Quetiapine: Use and Commercial Availability
Quetiapine treats the symptoms of schizophrenia. Furthermore, it may be used as monotherapy or in combination with other medicines to treat acute manic or mixed episodes in people with bipolar l disorder, bipolar disorder, and major depressive disorder. It treats depressive episodes in people. This drug is available in the market under the brand name Seroquel.
Quetiapine Structure and Mechanism of Action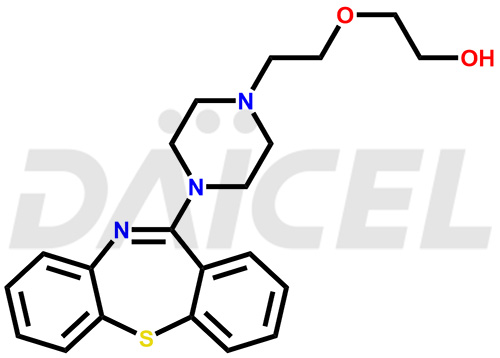
The chemical name of Quetiapine is 2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol. Its chemical formula is C21H25N3O2S, and its molecular weight is approximately 383.5 g/mol.
The mechanism of action of Quetiapine is not known.
Quetiapine Impurities and Synthesis
Quetiapine impurities are undesired compounds found in Quetiapine formulations that can occur during the synthetic process1 or manufacture or storage. They can impair Quetiapine’s quality, safety, and efficacy. As a result, stringent controls are put in place to identify, characterize, and manage impurities within permissible ranges.
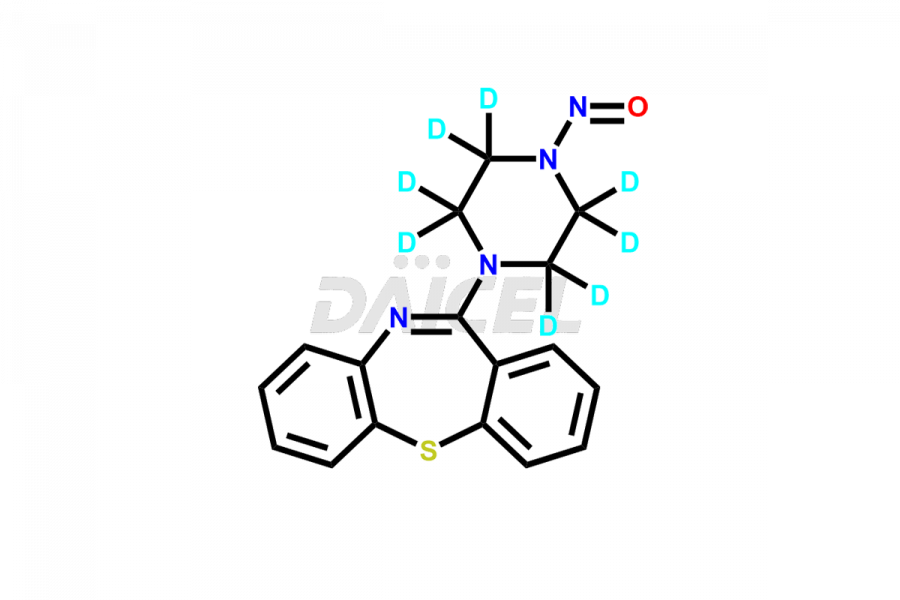
Daicel Pharma offers a Certificate of Analysis (CoA) for Quetiapine impurity standards such as Quetiapine Imp-A, Quetiapine Imp-O, Quetiapine Tetra Ethylene Glycol Fumarate Salt, Nitroso aryl piperazine, and 11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine. Our cGMP-certified analytical laboratory provides the CoA, which includes thorough characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give more characterization details, such as those for 13C-DEPT. At Daicel Pharma, our team of experts specializes in synthesizing Quetiapine impurities and labeled compounds for evaluating the effectiveness of generic Quetiapine. Additionally, Daicel Pharma offers Quetiapine.HCl-D4 and Nitroso aryl piperazine-D8, deuterium-labeled Quetiapine compounds, crucial for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Warawa, Edward John; Migler, Bernard Martin, Thiazepine compounds, ICI Americas, Inc., United States, EP0240228A1, October 7, 1987
- Mandrioli, R.; Fanali, S.; Ferranti, A.; Raggi, M. A., HPLC analysis of the novel antipsychotic drug quetiapine in human plasma, Journal of Pharmaceutical and Biomedical Analysis, Volume: 30, Issue: 4, Pages: 969-977, 2002
Frequently Asked Questions
Are there any specific Quetiapine impurities for monitoring?
All Quetiapine impurities are monitored strictly during the process.
How are Quetiapine impurities detected during the manufacturing process?
Quetiapine impurities are examined during quality control testing by employing analytical techniques to identify and quantify the impurities present in the Quetiapine.
What are the temperature conditions required to store Quetiapine impurities?
Quetiapine impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.

![11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine](https://dev.daicelpharmastandards.com/wp-content/uploads/2022/04/DCTI-C-1686-2-900x600.jpg)