Pyridostigmine
General Information
Pyridostigmine Impurities and Pyridostigmine
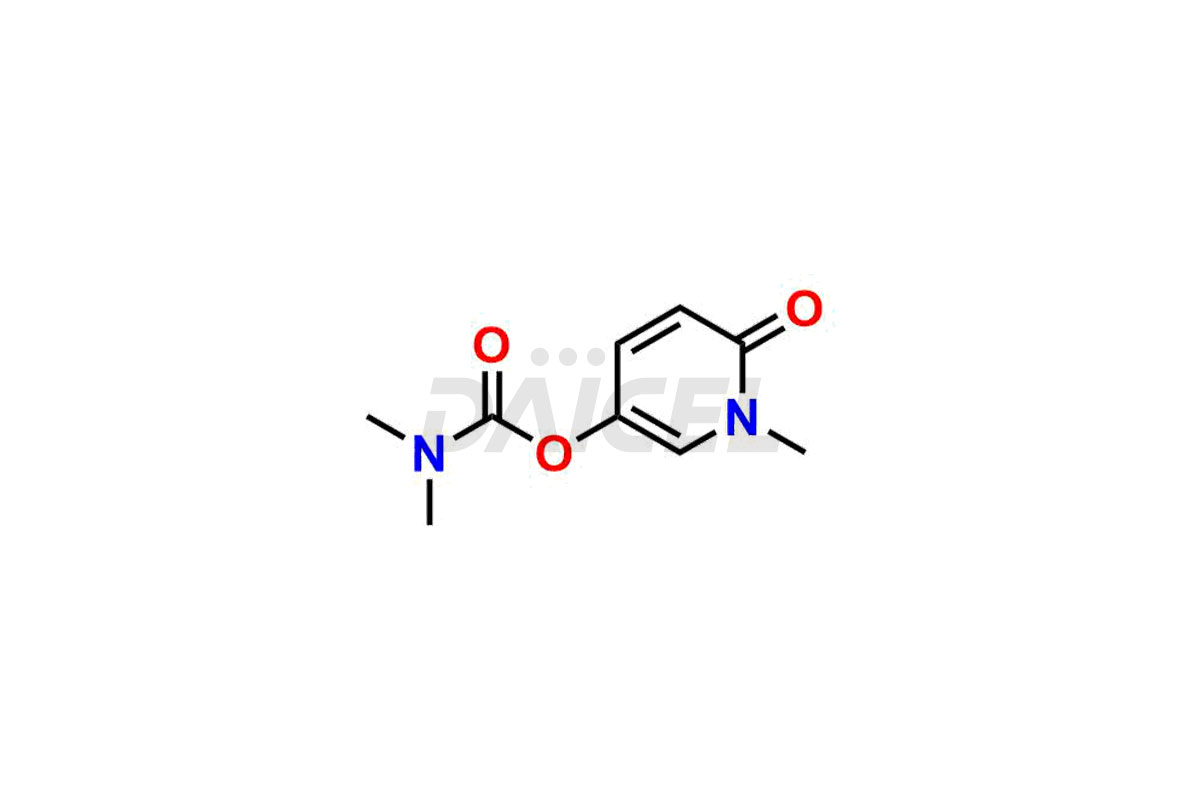
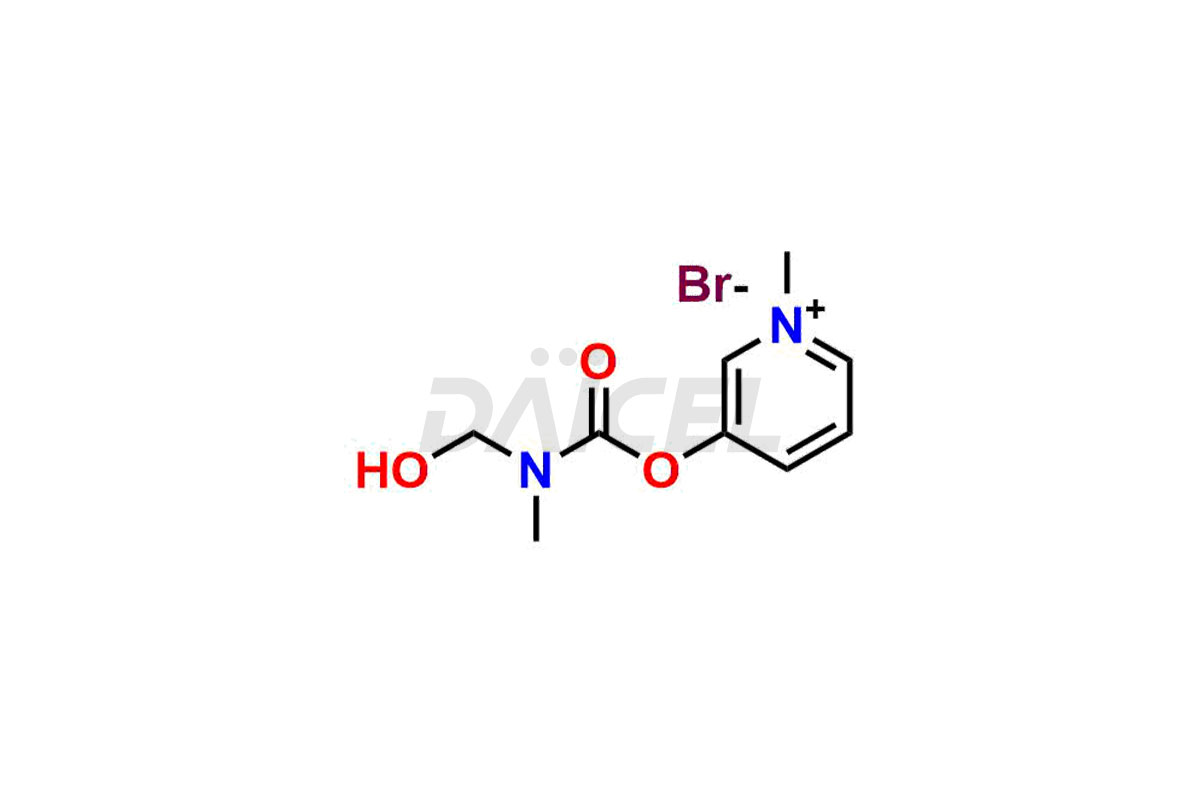
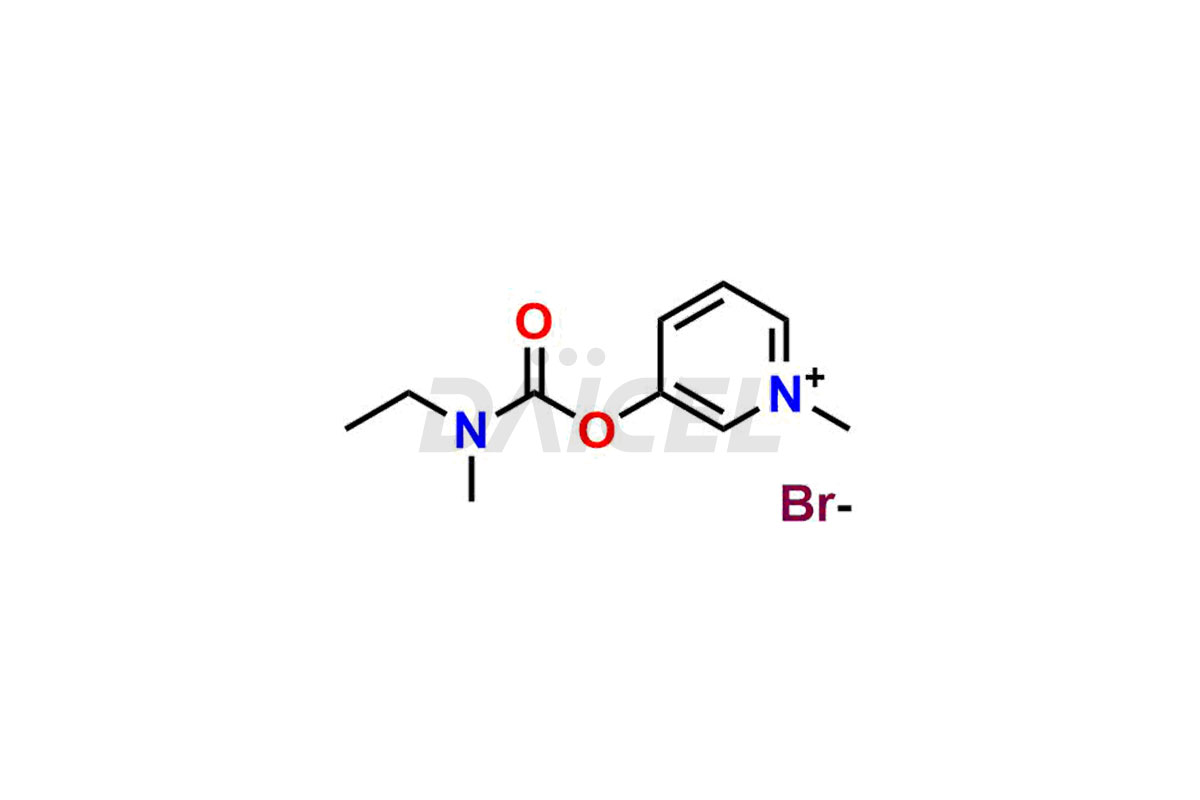
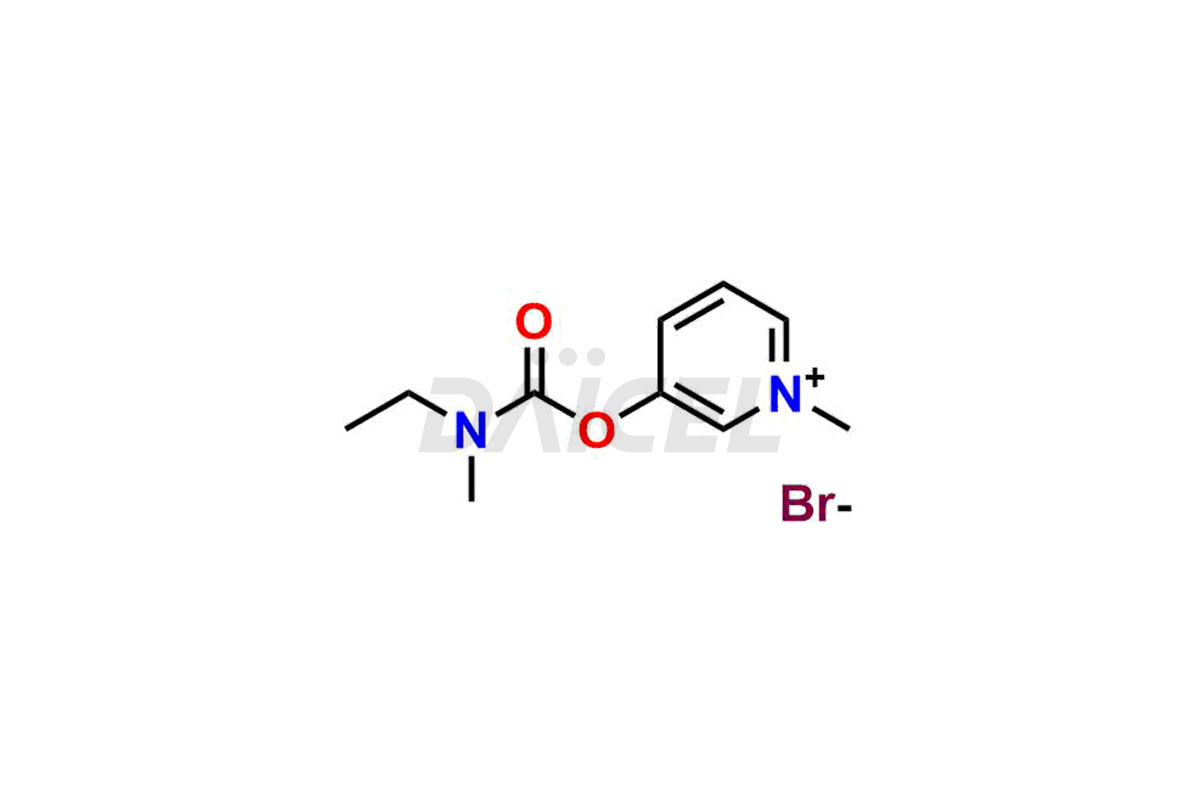
Daicel Pharma offers Pyridostigmine impurity standards which include Pyridostigmine EP impurity B, 3-((ethyl(methyl)carbamoyl)oxy)-1-methylpyridin-1-ium bromide, 1-Methyl-6-oxo-1,6-dihydropyridin-3-yl dimethyl carbamate and 3-(((hydroxymethyl)(methyl)carbamoyl)oxy)-1-methylpyridin-1-ium bromide. They are crucial to assess the purity, quality, and safety of Pyridostigmine. Furthermore, Daicel Pharma offers custom synthesis of Pyridostigmine impurities and delivers them globally.
Pyridostigmine [CAS: 155-97-5] is a US FDA-approved medicine to treat myasthenia gravis.
Pyridostigmine: Use and Commercial Availability
Pyridostigmine treats myasthenia gravis and counteracts the effects of muscle relaxants. This drug is available under the tradenames Mestinon and Regonol.
Pyridostigmine Structure and Mechanism of Action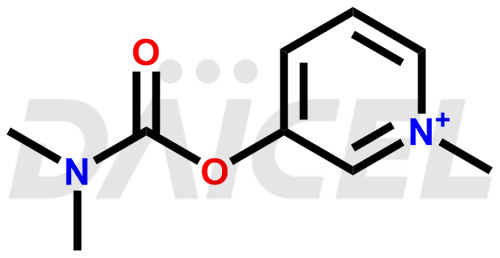
The chemical name of Pyridostigmine is 3-[[(Dimethylamino)carbonyl]oxy]-1-methylpyridinium. Its chemical formula is C9H13N2O2, and its molecular weight is approximately 181.21 g/mol.
Pyridostigmine Impurities and Synthesis
Pyridostigmine impurities can arise during the drug’s manufacture, storage, or use due to causes such as adverse effects and degradation. Analytical procedures such as HPLC evaluate and quantify these impurities. Control strategies include improving synthesis conditions, and purification techniques may aid in reducing these impurity levels in the drug.
Daicel Pharma provides a Certificate of Analysis (CoA) for Pyridostigmine impurity standards which include Pyridostigmine impurity impurities which include Pyridostigmine EP impurity B, 3-((ethyl(methyl)carbamoyl)oxy)-1-methylpyridin-1-ium bromide, 1-Methyl-6-oxo-1,6-dihydropyridin-3-yl dimethyl carbamate and 3-(((hydroxymethyl)(methyl)carbamoyl)oxy)-1-methylpyridin-1-ium bromide. At Daicel Pharma, we operate a cGMP-certified analytical laboratory that provides the CoA, which includes thorough characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity1 ,2. On request, we give more characterization details, such as 13C-DEPT, can be given. Daicel Pharma’s expert team prepares Pyridostigmine impurities.
References
FAQ's
References
- Yakatan, Gerald J.; Tien, Jy-Yean, Quantitation of pyridostigmine in plasma using high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications,Volume: 164 Issue: 3, Pages: 399-403,1979
- Coper, H.; Deyhle, G.; Dross, K., Absorption of pyridostigmine. Application of a spectrophotometric method for the determination of pyridostigmine in plasma, Zeitschrift fuer Klinische Chemie und Klinische Biochemie, Volume: 12, Issue: 6, Pages: 273-5, 1974
Frequently Asked Questions
Are the levels of Pyridostigmine impurities strictly regulated?
Yes, the levels of Pyridostigmine impurities are typically regulated to ensure the quality and safety of the drug.
What are the steps to remove Pyridostigmine impurities during the synthesis process?
During the synthetic process of Pyridostigmine, impurities are removed through purification methods such as crystallization, chromatography, or recrystallization.
What are the measures to ensure the safety and quality of Pyridostigmine impurities?
Rigorous testing and analysis are conducted throughout the manufacturing process, utilizing methods such as chromatography and spectroscopy to ensure the safety and quality of Pyridostigmine impurities and accurately identify, quantify, and control impurities within acceptable limits set by regulatory authorities.
What are the temperature conditions required to store Pyridostigmine impurities?
Pyridostigmine impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.