Propofol
General Information
Propofol Impurities and Propofol
Daicel Pharma offers Propofol impurity standards, which include Propofol EP impurity-K, Propofol EP impurity-L, and Propofol EP impurity-O. Their presence can influence the effectiveness, stability, and safety of Propofol. Daicel Pharma offers custom synthesis for Propofol impurities and provides international delivery.
Propofol [CAS: 2078-54-8] is a phenol that acts as an anesthetic and for managing refractory status epilepticus.
Propofol: Use and Commercial Availability
Propofol is an anesthetic drug that is injected intravenously during general anesthesia. It acts as a sedative and an anticonvulsant. It also keeps patients in a coma in critical care units (ICUs). This drug is available under tradenames such as Diprivan.
Propofol Structure and Mechanism of Action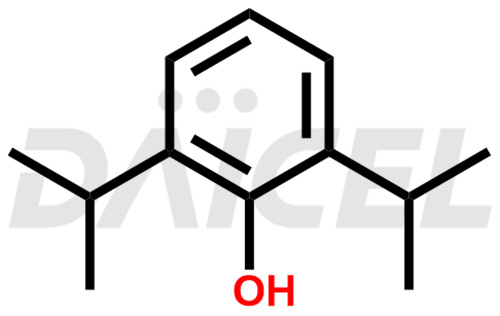
The chemical name of Propofol is 2,6-Bis(1-methylethyl)phenol. Its chemical formula is C12H18O, and its molecular weight is approximately 178.27 g/mol.
Propofol inhibits the functioning of the neurotransmitter gamma-aminobutyric acid (GABA).
Propofol Impurities and Synthesis
Impurities in Propofol are unwanted chemicals present as unexpected byproducts of the synthetic1 process, or they get introduced during production or storage. These impurities may influence the drug’s quality, safety, and efficacy.
Daicel Pharma offers a Certificate of Analysis (CoA) for Propofol impurity standards such as Propofol EP impurity-K, Propofol EP impurity-L, and Propofol EP impurity-O, from an analytical facility that is cGMP-compliant. Daicel Pharma provides a CoA with comprehensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization details, including 13C-DEPT, can be provided upon request. At Daicel Pharma, our team of experts can provide impurities and labeled compounds of Propofol to assess generic Propofol.
References
FAQ's
References
- Kolka, Alfred J.; Napolitano, John P.; Ecke, George G., The ortho alkylation of phenols, Journal of Organic Chemistry, Volume: 21, Pages: 712-13,1956
- Plummer, G. F., Improved method for the determination of propofol in blood by high-performance liquid chromatography with fluorescence detection, Journal of Chromatography, Biomedical Applications, Volume: 421, Issue: 1, Pages: 171-6, 1987
Frequently Asked Questions
What function does a cGMP-certified analytical laboratory play in detecting Propofol impurities?
A cGMP-certified analytical laboratory helps ensure the quality and safety of the drug while detecting Propofol impurities.
How can Propofol impurities appear in the drug?
Propofol impurities can arise in the drug as unintended byproducts of the synthetic process or as impurities introduced during manufacturing or storage.
Are Propofol impurities monitored in clinical settings?
Propofol impurities are monitored through various steps in clinical settings.
What are the temperature conditions required to store Propofol impurities?
Propofol impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.