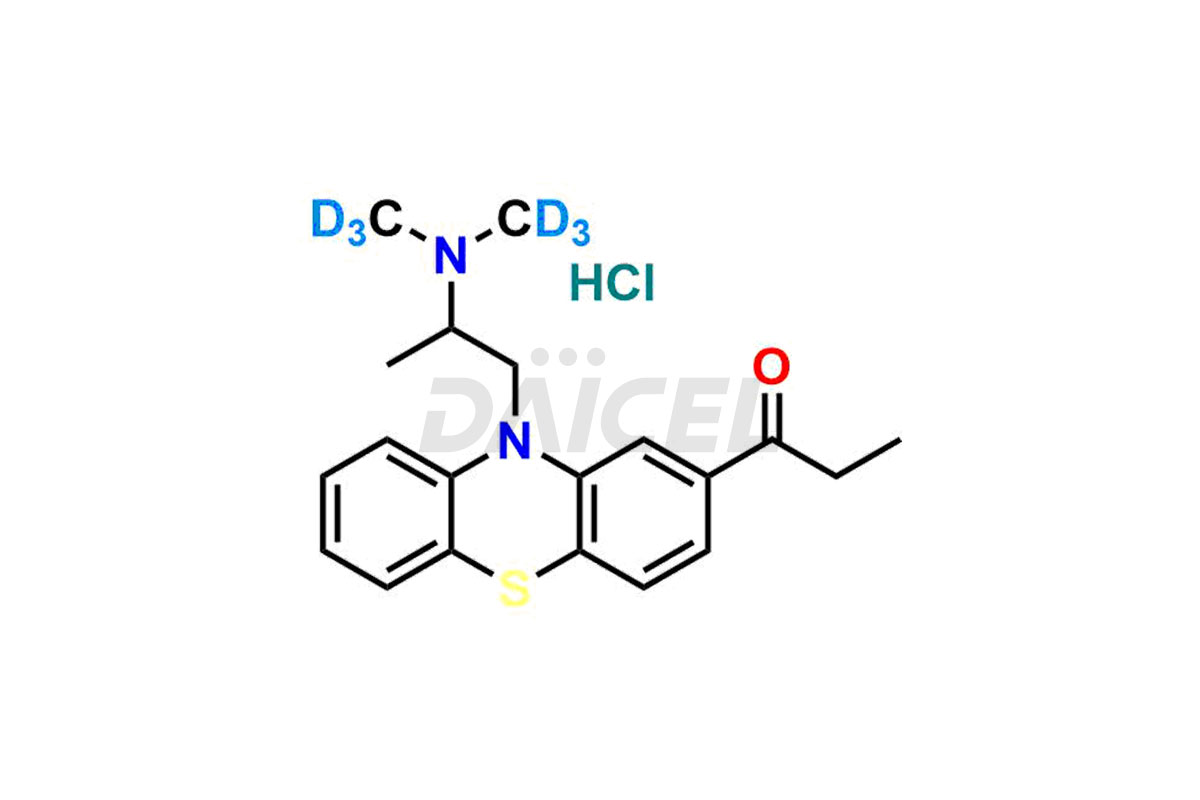
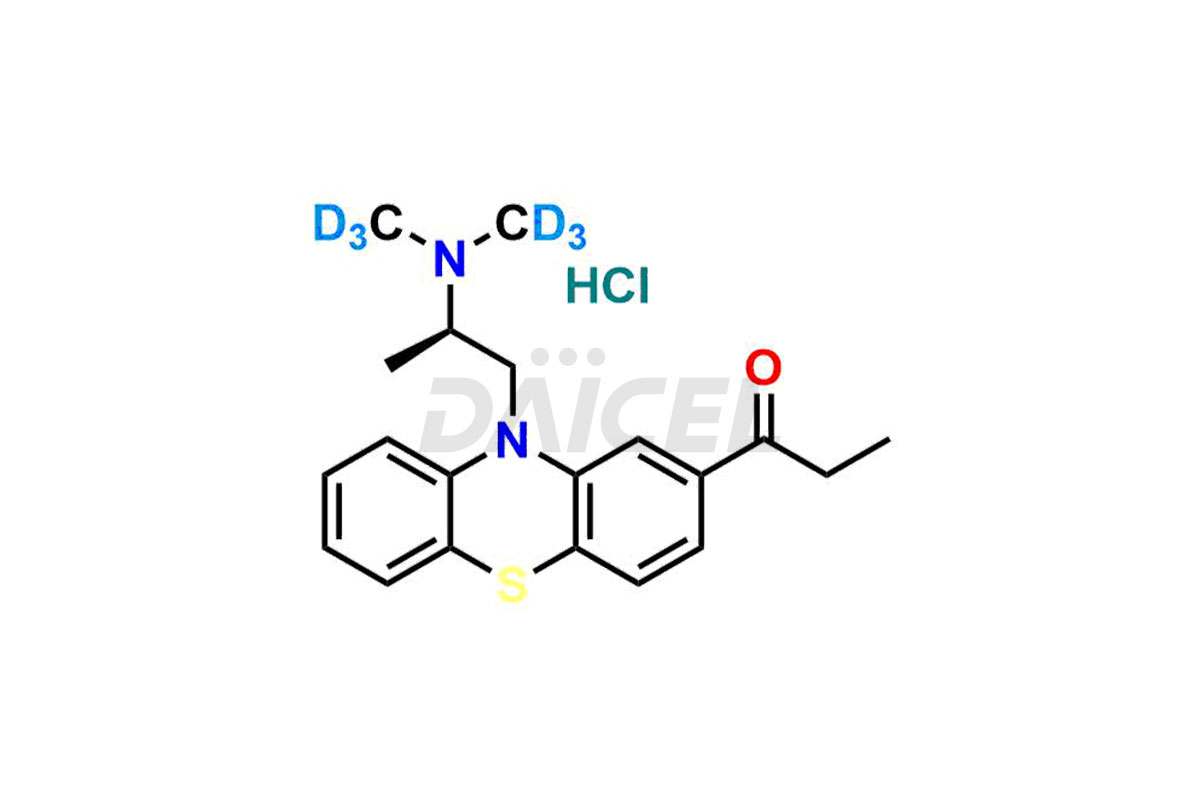
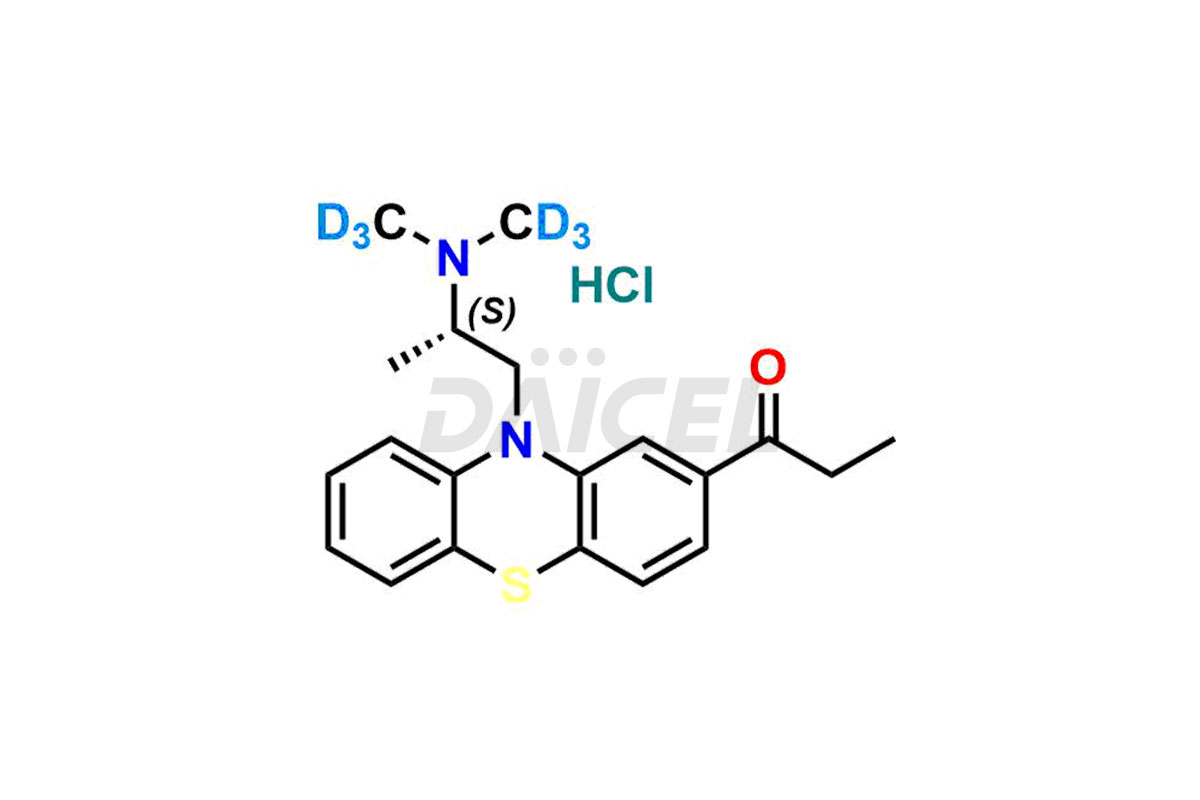
Propiomazine
General Information
Propiomazine Impurities and Propiomazine
Daicel Pharma synthesizes high-graded Propiomazine impurity standards. Their presence impacts the drug’s stability and causes potential adverse effects. Daicel Pharma offers a customized synthesis of Propiomazine impurities to cater to individual client requirements, with worldwide delivery options available.
Propiomazine [CAS: 362-29-8] belongs to the phenothiazine family of compounds, specifically a 10H-phenothiazine derivative. Propiomazine treats schizophrenia symptoms, agitation, and sedative.
Propiomazine: Use and Commercial Availability
Propiomazine treats insomnia due to its antihistamine-induced sleep-indulging properties. Propiomazine is a phenothiazine and an atypical antipsychotic. It has antihistamine properties and is a sedative in treating insomnia. This drug is available under the tradenames of Propavan, Largon, etc.
Propiomazine Structure and Mechanism of Action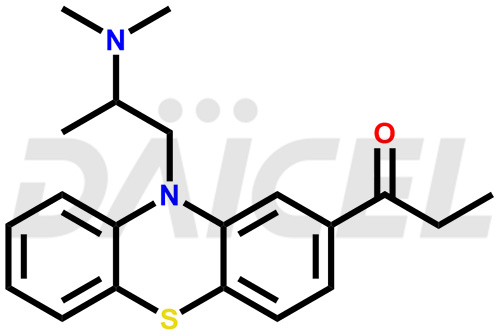
The chemical name of Propiomazine is 1-[10-[2-(Dimethylamino)propyl]-10H-phenothiazin-2-yl]-1-propanone. Its chemical formula is C20H24N2OS, and its molecular weight is approximately 340.5 g/mol.
Propiomazine is an antagonist of dopamine receptors 1,2,3 and 4.
Propiomazine Impurities and Synthesis
The production1 and storage of Propiomazine can lead to the formation of impurities, which can affect its purity and effectiveness. It ensures the purity of the initial components and carefully controls the reaction conditions during the synthetic process to minimize impurity formation. Impurities in Propiomazine can arise from side reactions, incomplete reactions, or contaminants in the starting materials.
Daicel Pharma provides a Certificate of Analysis (CoA) for Propiomazine impurity standards. Our analytical laboratory that meets cGMP requirements generates the Certificate of Analysis (CoA) with complete information, including extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Further characterization data, such as 13C-DEPT, can be supplied upon request. Our team of professionals at Daicel Pharma specializes in providing Propiomazine impurities and labeled compounds for analyzing the efficacy of generic Propiomazine. Additionally, Daicel Pharma offers Propiomazine-D6 Hydrochloride, R-Propiomazine-D6 Hydrochloride, and S-Propiomazine-D6 Hydrochloride, deuterium-labeled Propiomazine compounds, crucial for bio-analytical research, including BA/BE investigations. Every delivery comes with a detailed characterization report.
References
FAQ's
References
- Farbenfabriken Bayer Akt.-Ges., Phenothiazine derivatives, GB808049A, January 28, 1959
- Hartvig, Per; Aahs, Ulla; Wickstroem, Goeran, Determination of propiomazine and its N-demethyl metabolite in plasma by gas chromatography with alkali flame ionization detection, Journal of Chromatography, Biomedical Applications, Volume: 183, Issue: 2, Pages: 229-33, 1980
Frequently Asked Questions
Can Propiomazine impurities cause batch rejection or recall?
Yes, if the levels of Propiomazine impurities exceed regulatory limits or if they pose significant health risks, it can lead to batch rejection or recall of the product.
Which solvent helps analyze Propiomazine impurities?
The solvent commonly used to analyze Propiomazine impurities is a mixture of water and organic solvents, such as methanol or acetonitrile.
How can Propiomazine impurities influence the drug's bioavailability?
Propiomazine impurities can alter the drug's bioavailability by affecting its absorption, distribution, metabolism, or excretion processes.
What are the temperature conditions required to store Propiomazine impurities?
Propiomazine impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.