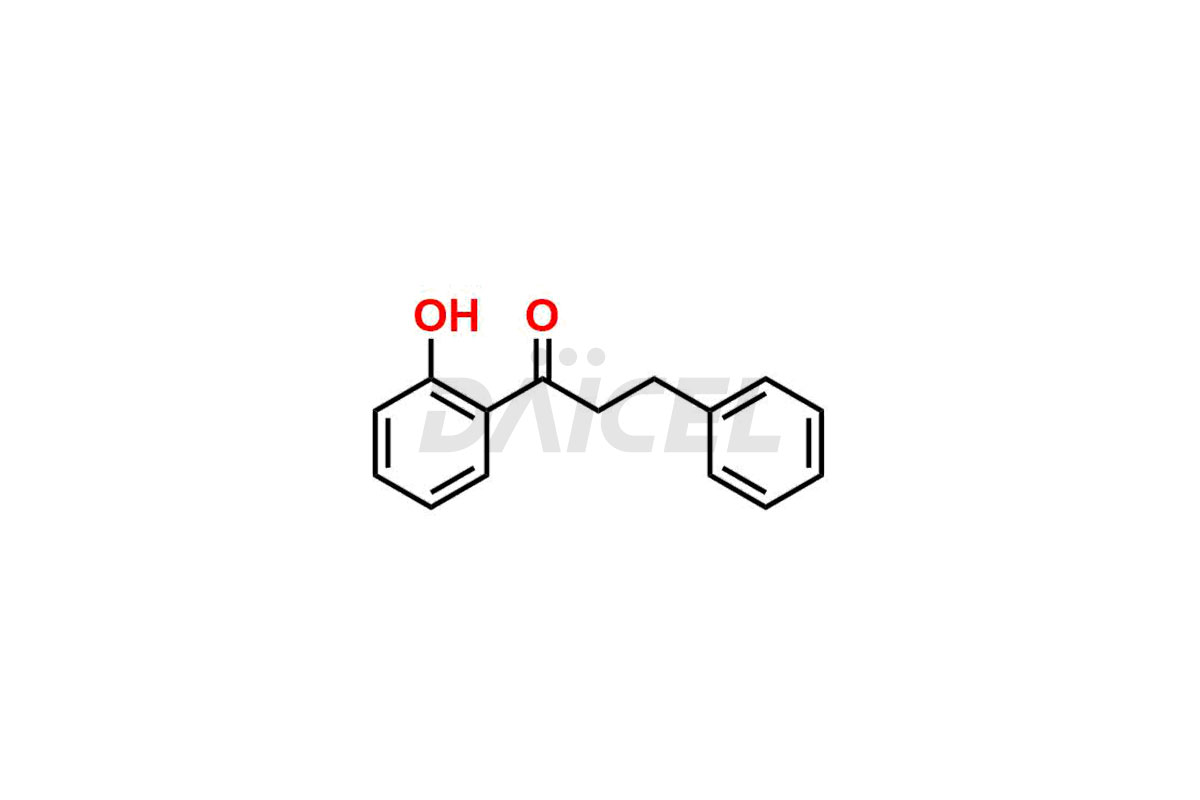
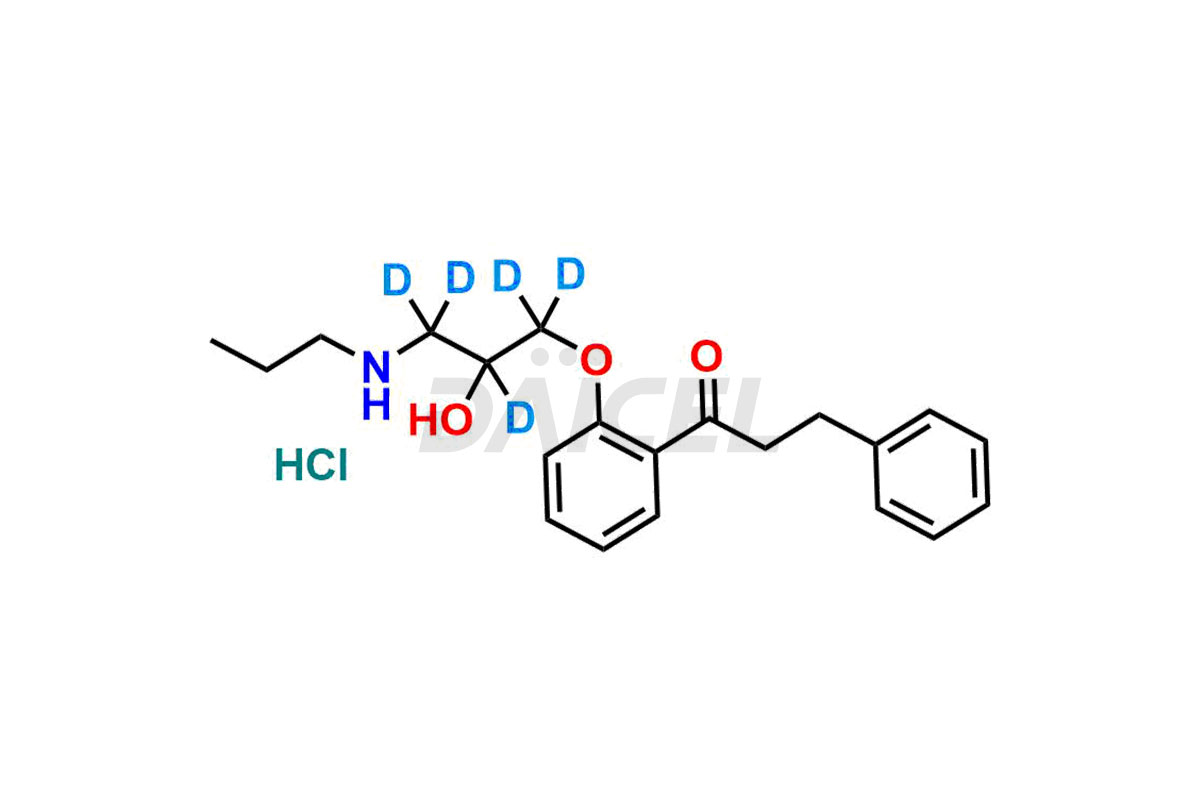
Propafenone
General Information
Propafenone Impurities and Propafenone
Daicel Pharma offers Propafenone impurity standards, which include Propafenone EP impurity A and Propafenone EP impurity C. These impurities are crucial to assess Propafenone purity, quality, and safety. Furthermore, Daicel Pharma provides custom synthesis of Propafenone impurities and delivers them globally.
Propafenone [CAS: 54063-53-5] is an aromatic ketone structure that belongs to the class 1C antiarrhythmic drugs and exhibits local anesthetic properties. Propafenone manages supraventricular and ventricular arrhythmias. It functions primarily as an anti-arrhythmia drug.
Propafenone: Use and Commercial Availability
Propafenone is prescribed to patients without structural heart disease to delay the reoccurrence of paroxysmal atrial fibrillation/flutter (PAF) associated distressing symptoms. It manages life-threatening documented ventricular arrhythmias, including sustained ventricular tachycardia. This drug is available under various brands Rythmol and Rythmol SR.
Propafenone Structure and Mechanism of Action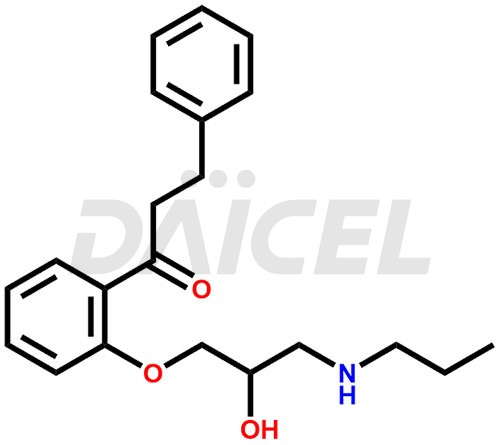
Impurities The chemical name of Propafenone is 1-[2-[2-Hydroxy-3-(propylamino)propoxy]phenyl]-3-phenyl-1-propanone. Its chemical formula is C21H27NO3, and its molecular weight is approximately 341.4 g/mol.
Propafenone has a direct stabilizing action on myocardial membranes. It depresses triggered activity and reduces spontaneous automaticity.
Propafenone Impurities and Synthesis
Impurities in Propafenone can form during its manufacture1 and storage, impacting its purity and efficacy. During the synthetic process, it is critical to guarantee the purity of the starting components while also managing reaction conditions to minimize impurity production. Impurities in Propafenone can be due to side reactions, incomplete reactions, or contaminants in the starting ingredients. Adequate purifying processes, such as crystallization and distillation, eliminate these impurities and generate a pure version of Propafenone.
Daicel Pharma offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Propafenone impurity standards such as Propafenone EP impurity A and Propafenone EP impurity C. At Daicel Pharma, we have a cGMP-certified analytical laboratory that provides the CoA, which includes thorough characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give more characterization details, such as those for 13C-DEPT. We offer Propafenone impurities and labeled compounds to evaluate generic Propafenone. Furthermore, we offer highly Propafenone-d5 Hydrochloride, a pure deuterium-labeled Propafenone compound that supports bioanalytical research and Bioavailability/Bioequivalence (BA/BE) research.
References
FAQ's
References
- Lietz, Helmut, Preparation of propafenone, BASF A.-G., Federal Republic of Germany, US4474986A, October 2, 1984
- Harapat, Sandra R.; Kates, Robert E., High-performance liquid chromatographic analysis of propafenone in human plasma samples, Journal of Chromatography, Biomedical Applications Volume: 230, Issue: 2, Pages: 448-53, 1982
Frequently Asked Questions
What is the relevance of Propafenone impurity profiling?
The relevance of Propafenone impurity profiling is to ensure the purity, safety, and efficacy of the drug by identifying, quantifying, and controlling impurities that may be present in the formulation.
How are impurities in Propafenone detected and quantified?
Impurities in Propafenone are detected and quantified using analytical techniques such as high-performance liquid chromatography (HPLC) coupled with various detection methods such as mass spectrometry (MS).
What are the risks associated with certain Propafenone impurities?
Some impurities in Propafenone may pose risks such as decreased efficacy, increased toxicity, or potential for adverse reactions and allergies.
What are the temperature conditions required to store Propafenone impurities?
Propafenone impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.