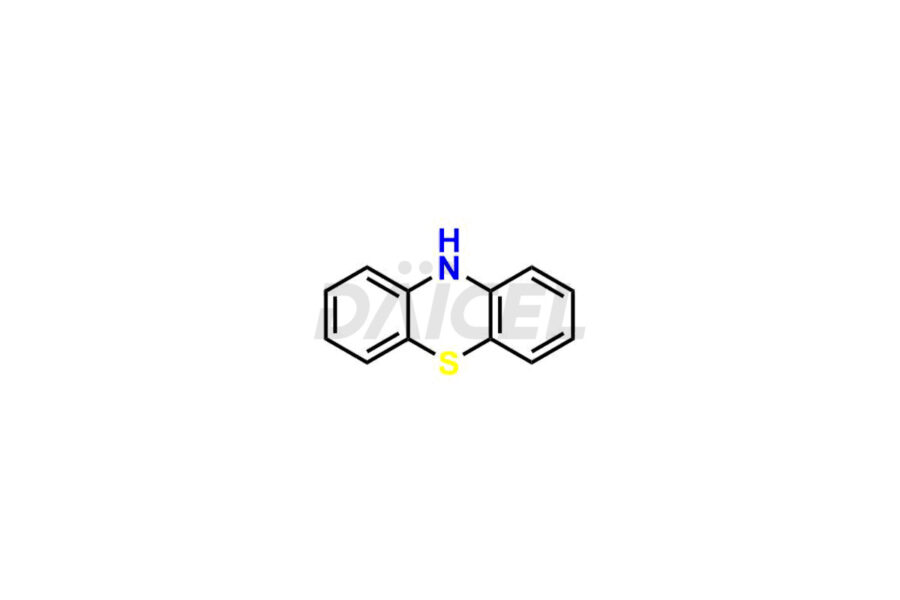
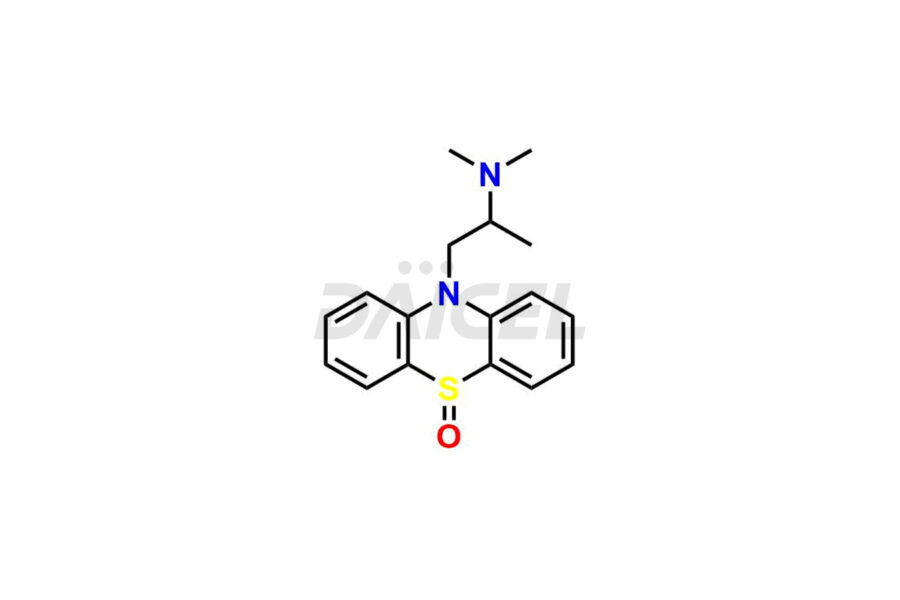
Promethazine
General Information
Promethazine Impurities and Promethazine
Daicel Pharma offers high-graded Promethazine impurity standards, which include Promethazine EP Impurity A and Promethazine sulfoxide impurity. These impurities can impact the efficacy, quality, and safety of Promethazine. Daicel Pharma provides a custom synthesis of Promethazine impurities to meet specific client needs, offering global delivery options for convenience.
Promethazine [CAS: 60-87-7] is an N-dimethyl aminopropyl derivative of phenothiazine. Promethazine treats allergies, motion sickness, nausea, and vomiting.
Promethazine: Use and Commercial Availability
Promethazine belongs to the phenothiazine class of compounds and treats Rhinitis, allergic conjunctivitis, allergic responses to blood or plasma, and dermographism. It is for promethazine cough syrup with phenylephrine and codeine to treat cold and allergy-related nasal congestion and cough and upper respiratory symptoms. This drug is available under various brand names such as Phenergan, Promethacon, Remsed, etc.
Promethazine Structure and Mechanism of Action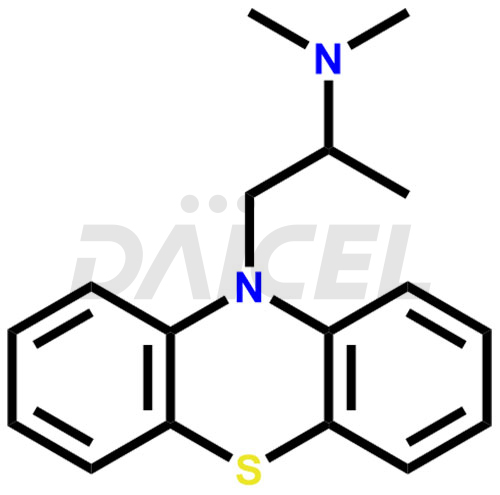
The chemical name of Promethazine is N, N, α-Trimethyl-10H-phenothiazine-10-ethanamine. Its chemical formula is C17H20N2S, and its molecular weight is approximately 284.4 g/mol.
Promethazine treats allergic reactions due to its antihistamine properties.
Promethazine Impurities and Synthesis
Promethazine, a versatile compound with various therapeutic properties, may contain impurities that can impact its purity and efficacy. During the synthetic process1, it ensures the purity of starting materials and the appropriate reaction conditions to minimize impurity formation. Impurities in Promethazine can arise from side reactions, incomplete reactions, or contaminants in the starting materials. Purification techniques such as filtration, recrystallization, or chromatography help remove these impurities and obtain a highly pure form of Promethazine.
Daicel Pharma provides a Certificate of Analysis (CoA) for Promethazine impurity standards, which include Promethazine EP Impurity A and Promethazine sulfoxide impurity. Our CoA from a cGMP-certified analytical laboratory includes thorough characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give more characterization details, such as those for 13C-DEPT. At Daicel Pharma, our team of experts specializes in offering Promethazine impurities and labeled compounds for evaluating the effectiveness of generic Promethazine. Additionally, Daicel Pharma offers Promethazine Labelled Standard, a deuterium-labeled Promethazine R&S isomer D6 compounds, crucial for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) research.
References
FAQ's
References
- Cusic, John W., 8-Haloxanthine Salts Of Aminoalkyl Phenothiazines And The Production thereof, G.D. Searle and Co., US2534237A, December 19, 1950
- Hulshoff, Abram; Perrin, John H., Chromatographic characterization of phenothiazine drugs by a reversed-phase thin-layer technique, Journal of Chromatography, Volume: 129, Pages: 249-62, 1976
Frequently Asked Questions
How can Promethazine impurities be kept to a minimum while stored?
Proper storage of Promethazine from light, moisture, and extreme temperature, can reduce impurity accumulation over time. Following the manufacturer's or regulatory bodies' suggested storage parameters is critical to maintaining the drug's purity.
Are there specific Promethazine impurities that are common?
Impurities in Promethazine might vary based on the manufacturing process and starting ingredients. They include related compounds that might occur during synthesis, breakdown products, residual solvents, and handling or storage.
Can Promethazine impurities alter over time?
Promethazine's chemical makeup can degrade over time or combine with other chemicals causing new impurity formation. Proper Promethazine storage and handling can help reduce impurity formation and maintain the drug's stability.
What are the temperature conditions required to store Promethazine impurities?
Promethazine impurities are stored preferably at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.