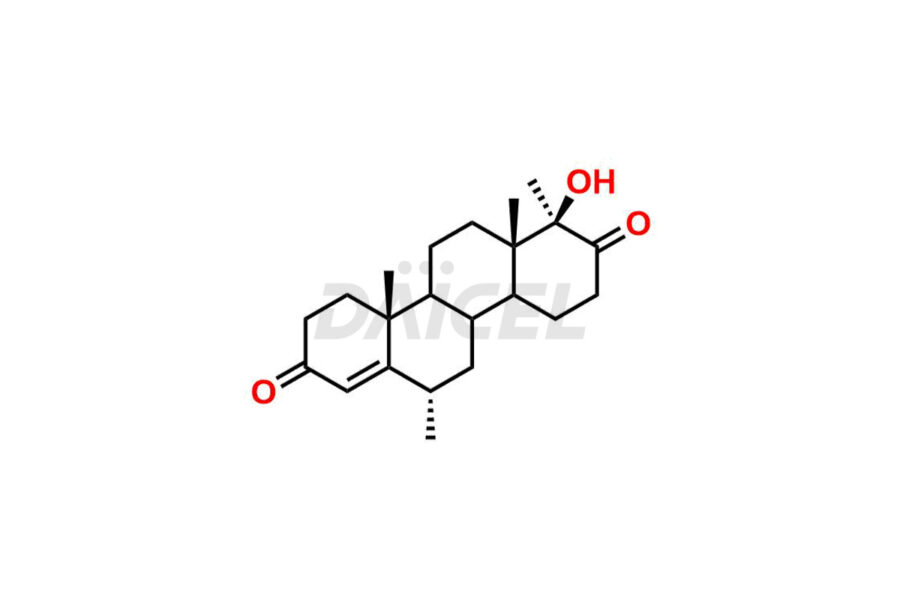
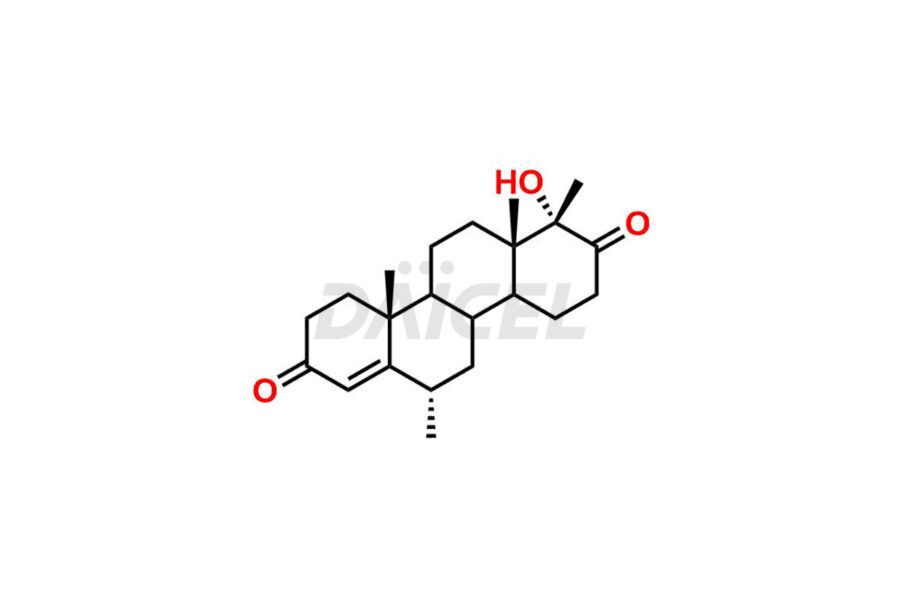
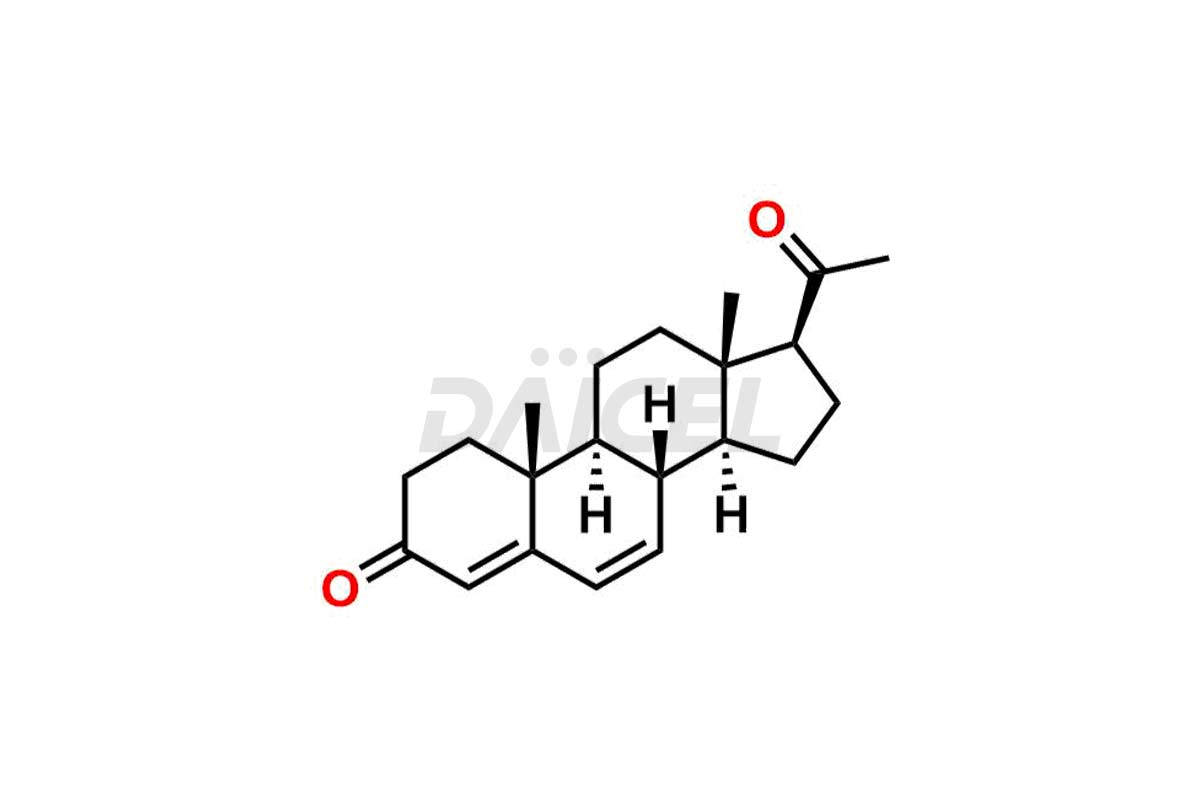
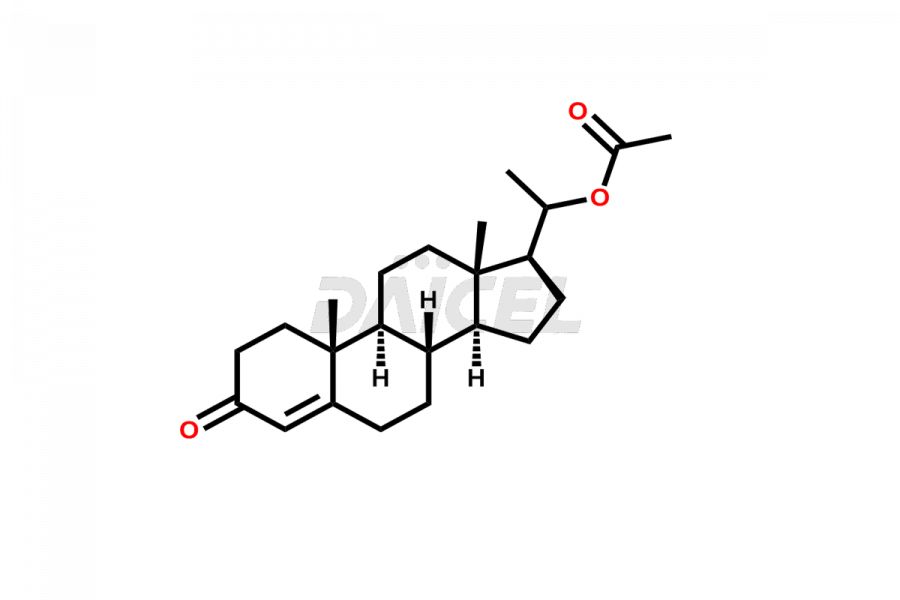
Progesterone
General Information
Progesterone Impurities and Progesterone
Daicel Pharma offers high-quality Progesterone impurity standards, 17α-Methyl-17-Keto-D-Homo medroxyprogesterone, 17α-Methyl-17-Keto-D-Homo medroxyprogesterone, 6-Dehydroprogesterone, Progesterone Impurity C, and Progesterone impurity D and E, which is crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Progesterone. Moreover, Daicel Pharma offers custom synthesis of Progesterone impurities and delivers them globally.
Therapeutic Progesterone [CAS: 57-83-0], a synthetic version of the hormone progesterone, prevents ovulation and fertilization in various contraceptive preparations. Additionally, Progesterone induces changes in the endometrium, decreases uterine contractility during pregnancy, and supports the maintenance of pregnancy.
Progesterone: Use and Commercial Availability
Progesterone is available in various forms, including gelatinized capsules, vaginal gel, vaginal inserts, injections, and contraception tablets. Gelatinized capsules prevent endometrial hyperplasia and treat secondary amenorrhea. Vaginal gel, available in different concentrations, is used in assisted reproductive treatments and for secondary amenorrhea. Vaginal inserts support embryo implantation and early pregnancy. Injections are for amenorrhea and abnormal uterine bleeding, and progesterone tablets act as contraceptives. Some brand names for Progesterone include Crinone, Endometrin, Milprosa, Progestasert, etc.
Progesterone Structure and Mechanism of Action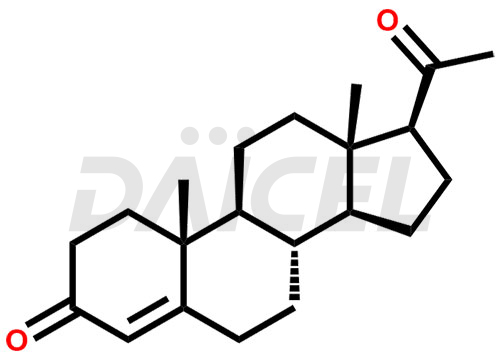
The chemical name of Progesterone is Pregn-4-ene-3,20-dione. Its chemical formula is C21H30O2, and its molecular weight is approximately 314.5 g/mol.
Progesterone binds to progesterone receptors, leading to the activation of transcription through interactions with transcription factors. It exerts inhibition on estrogens by reducing estrogen receptors and increasing estrogen metabolism.
Progesterone Impurities and Synthesis
Impurities can form during the synthesis1, purification, and storage of Progesterone. They can arise from various sources, such as starting materials, reaction intermediates, and degradation products. Controlling the impurity profile of Progesterone is crucial for ensuring its safety and efficacy.
Daicel Pharma offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Progesterone impurity standards such as 17α-Methyl-17-Keto-D-Homo medroxyprogesterone, 17α-Methyl-17-Keto-D-Homo medroxyprogesterone, 6-Dehydroprogesterone, Progesterone Impurity C, and Progesterone impurity D and E. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT. Upon delivery, we give a complete characterization report. Daicel Pharma has the technology and expertise to prepare any unknown Progesterone impurity or degradation product.
References
FAQ's
References
- Sundararaman, Padmanabhan; Djerassi, Carl, A convenient synthesis of progesterone from stigmasterol, Journal of Organic Chemistry, Volume: 42, Issue: 22, Pages: 3633-4, 1977
- Wyman, Heather; Sommerville, Ian F., Description and evaluation of a simple technique for determination of plasma progesterone by thin-layer and gas-liquid chromatography, Steroids, Volume: 12, Issue: 1, Pages: 63-86, 1968
Frequently Asked Questions
How can impurities be removed from Progesterone?
Progesterone impurities can be removed from Progesterone through various purification methods, including recrystallization, column chromatography, and solvent extraction.
What is the impact of Progesterone impurities on the stability of the drug?
Progesterone impurities can affect the stability of Progesterone, leading to its degradation and reduced efficacy.
What is the impact of Progesterone impurities on the drug’s shelf life?
Progesterone impurities can shorten the shelf life of Progesterone by accelerating the degradation of the compound.
How can Progesterone impurities affect the bioavailability of the drug?
Chemical interactions between Progesterone impurities and the active ingredient can alter the stability and solubility of Progesterone, leading to decreased bioavailability and efficacy of the drug. They can also react with other components of the drug formulation, leading to the formation of insoluble complexes that reduce absorption and distribution.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.