LOAD MORE
You're viewed 9 of 29 products
Daicel Pharma synthesizes high-quality Pregabalin impurities, (S)-isopropyl 2-hydroxy-2-phenyl-acetate, (R)-isopropyl 2-hydroxy-2-phenyl-acetate, 3-(aminomethyl)-5-methylhex-5-enoic acid, dimethyl 3-isobutylpentanedioate, and more, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Pregabalin. Moreover, Daicel Pharma offers custom synthesis of Pregabalin impurities and delivers them globally.
Pregabalin [CAS: 148553-50-8] is a 3-isobutyl derivative of the neurotransmitter γ-aminobutyric acid. It has anticonvulsant properties. Pregabalin helps prevent seizures, reduces anxiety, and relieves pain. It also treats painful neuropathy and epilepsy.
Pregabalin treats neuropathic pain and seizures. It is available under the brand names LYRICA and LYRICA CR. It treats neuropathic pain associated with diabetic peripheral neuropathy, spinal cord injury, postherpetic neuralgia, and fibromyalgia. It also acts as an adjunctive therapy for partial-onset seizures in adults with epilepsy.
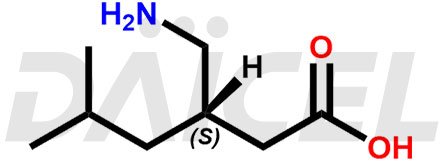
The chemical name of Pregabalin is (3S)-3-(Aminomethyl)-5-methylhexanoic acid. Its chemical formula is C8H17NO2, and its molecular weight is approximately 159.23 g/mol.
Pregabalin binds with the alpha2-delta site of presynaptic voltage-dependent calcium channels in central nervous system tissues.
Impurities may form during the manufacturing1, storage, or usage of Pregabalin. They include related substances, residual solvents, and degradation products. Some of these impurities may be toxic and impact the drug’s quality, safety, and efficacy. Therefore, it is essential to monitor and control the impurities in Pregabalin through analytical methods and proper storage conditions.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Pregabalin impurity standards, (S)-isopropyl 2-hydroxy-2-phenyl-acetate, (R)-isopropyl 2-hydroxy-2-phenyl-acetate, 3-(aminomethyl)-5-methylhex-5-enoic acid, dimethyl 3-isobutylpentanedioate, and more. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Pregabalin impurity or degradation product.
Impurity profiling is essential in identifying and controlling impurities in Pregabalin during manufacturing.
Various analytical methods like High-Performance Liquid Chromatography (HPLC), Liquid Chromatography-mass spectroscopy (LC-MS), etc., help identify impurities in Pregabalin.
Methanol or Acetonitrile is the solvent used for analyzing many impurities in Pregabalin.
Pregabalin impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.