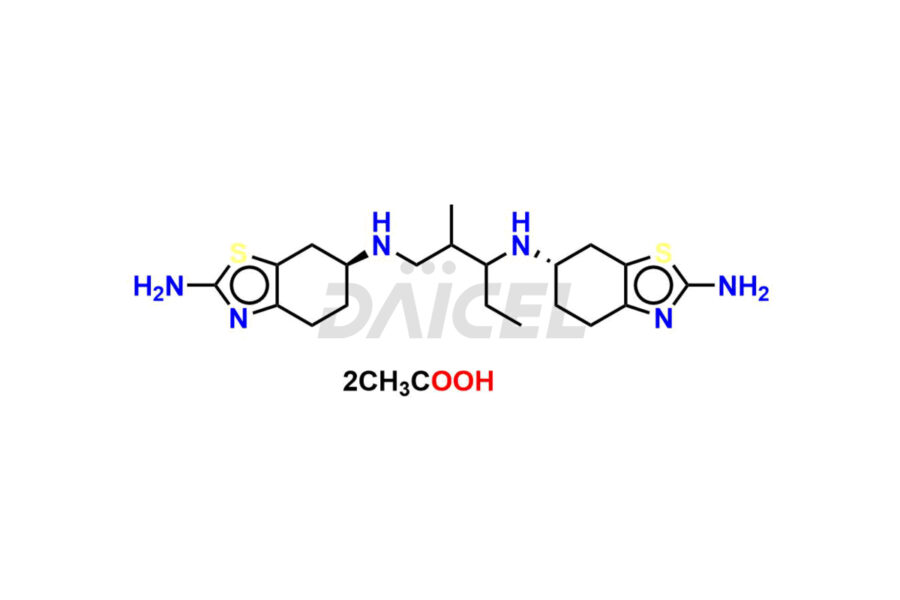
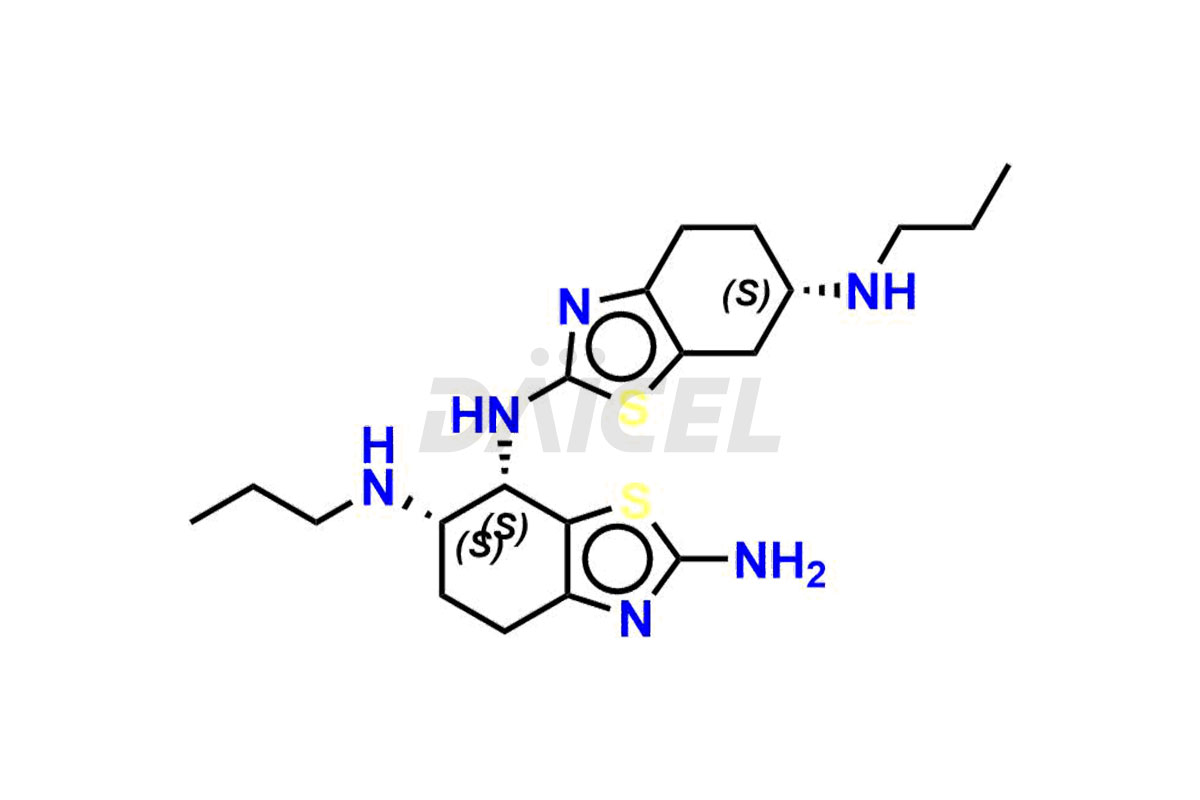
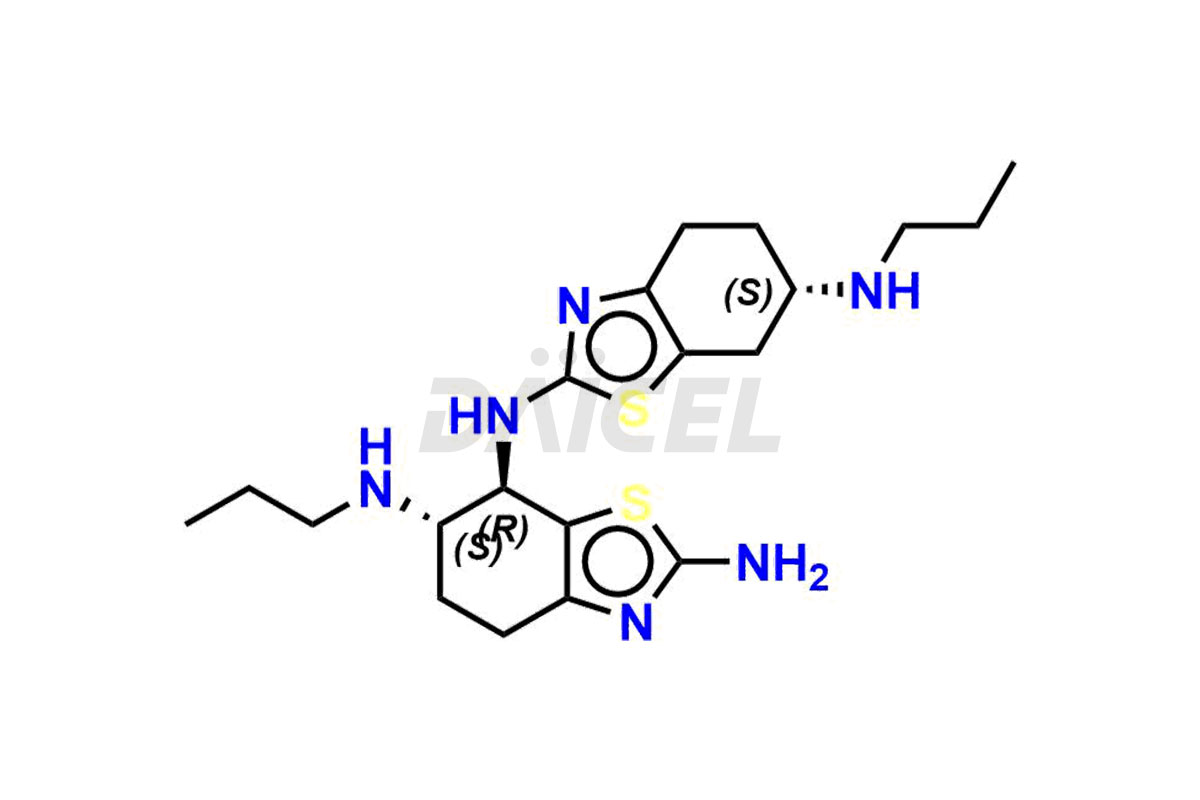
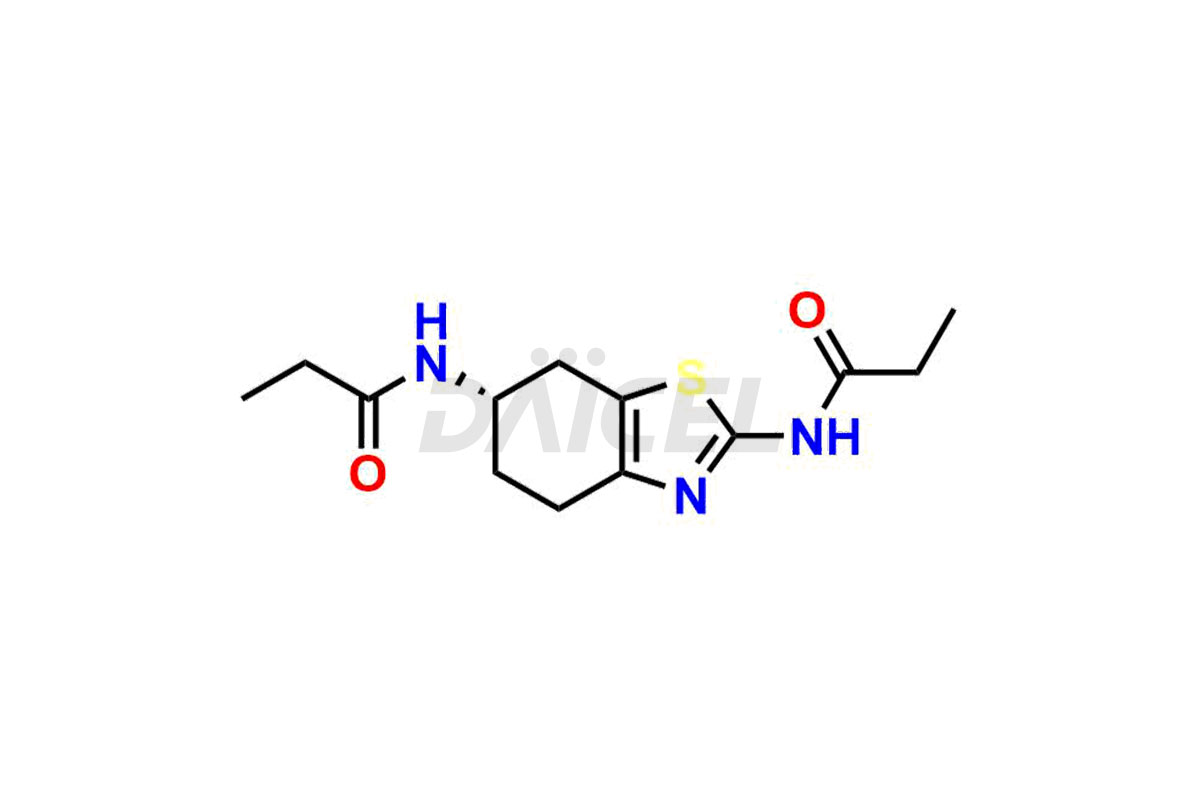
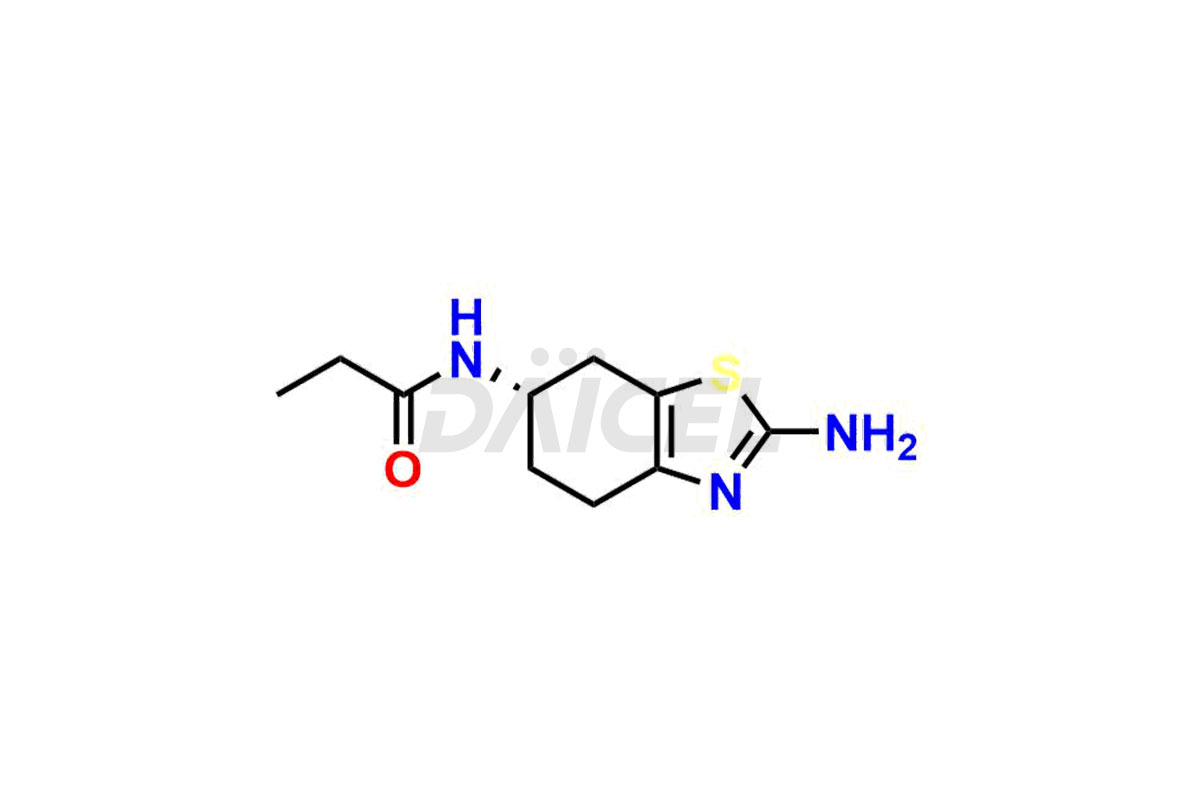
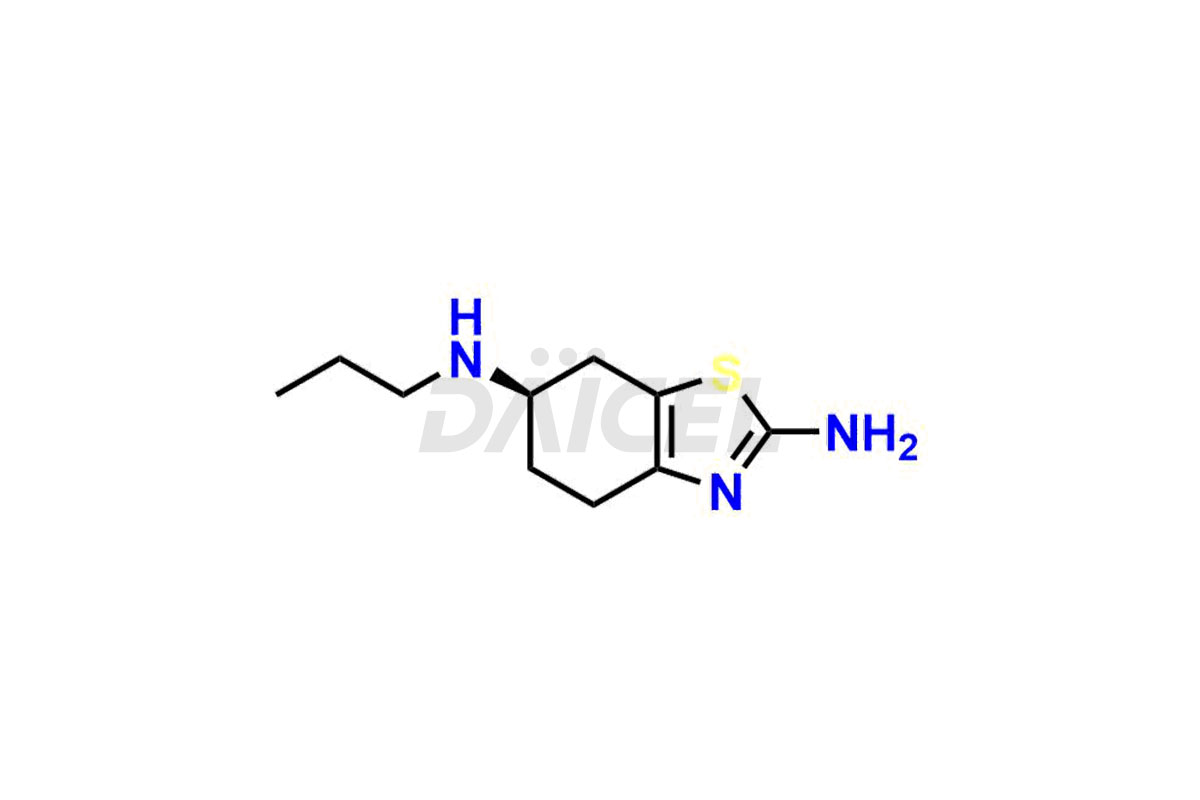
Pramipexole
General Information
Pramipexole Impurities and Pramipexole
Daicel Pharma synthesizes high-quality Pramipexole impurities, Pramipexole Dimer, Pramipexole dimer- I, Pramipexole dimer- II, Pramipexole impurity 29, Pramipexole impurity E (BP), and Pramipexole related compound D, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Pramipexole. Moreover, Daicel Pharma offers custom synthesis of Pramipexole impurities and delivers them globally.
Pramipexole [CAS: 104632-26-0] is a medicine that stimulates dopamine receptors. It treats Parkinson’s disease and restless leg syndrome. It belongs to the anti-parkinsonian class of drugs.
Pramipexole: Use and Commercial Availability
Pramipexole is a non-ergot-derived dopaminergic agonist that treats Parkinson’s disease and restless leg syndrome. It can be used as a monotherapy or add-on drug to other first-line agents for Parkinson’s disease. Pramipexole is under research for treating bipolar depression, treatment-resistant depression, etc. Pramipexole is available under brand names like Mirapex, Pramipexole Dihydrochloride, And Mirapex Er.
Pramipexole Structure and Mechanism of Action 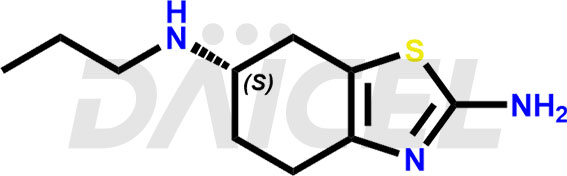
The chemical name of Pramipexole is (6S)-4,5,6,7-Tetrahydro-N6-propyl-2,6-benzothiazolediamine. Its chemical formula is C10H17N3S, and its molecular weight is approximately 211.33 g/mol.
Pramipexole has intrinsic activity at the D2 subfamily of dopamine receptors. It is a non-ergot dopamine agonist with a higher binding affinity to the D3 receptor subtype.
Pramipexole Impurities and Synthesis
Pramipexole impurities form during the synthesis1,2, purification, or storage of the drug. These impurities may include oxidation products, degradants from exposure to light or moisture, and residual solvents from the purification process. The formation of these impurities can affect the drug potency and safety, and therefore their levels must be carefully monitored and controlled during manufacturing and storage.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Pramipexole impurity standards, Pramipexole Dimer, Pramipexole dimer- I, Pramipexole dimer- II, Pramipexole impurity 29, Pramipexole impurity E (BP), and Pramipexole related compound D. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity3. We also provide 13C-DEPT and CHN on request. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Pramipexole impurity or degradation product.
References
FAQ's
References
- Griss, Gerhart; Schneider, Claus; Hurnaus, Rudolf; Kobinger, Walter; Pichler, Ludwig; Bauer, Rudolf; Mierau, Joachim; Hinzen, Dieter; Schingnitz, Guenter, Tetrahydro-benzthiazoles, the preparation thereof and their use as intermediate products or as pharmaceuticals, Thomae, Dr. Karl, G.m.b.H., Federal Republic of Germany, US4731374A, March 15, 1988
- Schneider, Claus S.; Mierau, Joachim, Dopamine autoreceptor agonists: resolution and pharmacological activity of 2,6-diaminotetrahydrobenzothiazole and an aminothiazole analog of apomorphine, Journal of Medicinal Chemistry, Volume: 30, Issue: 3, Pages: 494-8, 1987
- Bharathi, D. Vijaya; Hotha, Kishore Kumar; Sagar, P. V. Vidya; Kumar, S. Sirish; Naidu, A.; Mullangi, Ramesh, Development and validation of a sensitive LC-MS/MS method with electrospray ionization for quantitation of pramipexole in human plasma: application to a clinical pharmacokinetic study, Biomedical Chromatography, Volume: 23, Issue: 2, Pages: 212-218, 2009
Frequently Asked Questions
How can impurities in Pramipexole be minimized?
Impurities in Pramipexole are minimized by using top-quality starting materials, reagents, and solvents and refining the manufacturing process. Additionally, it is crucial to maintain appropriate storage conditions to prevent impurities from forming.
Can impurities in Pramipexole affect its bioavailability?
Impurities in Pramipexole affect its bioavailability by altering its pharmacokinetics. For example, few impurities are more readily absorbed by the body than the API, leading to a change in drug concentration and efficacy.
Which solvent help in the analysis of Pramipexole impurities?
Methanol is the solvent used in analyzing many Pramipexole impurities.
What are the temperature conditions required to store Pramipexole impurities?
Pramipexole impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.