LOAD MORE
You're viewed 9 of 17 products
Daicel Pharma synthesizes more than ten high-quality Posaconazole impurities, such as Posaconazole Impurity-(component-D), Posaconazole Succinyl Ester, Posaconazole Piperazine dioxide, Posaconazole impurity-B, Posaconazole Impurity 57, Desethylene Posaconazole N-formyl, Desethylene Posaconazole, and more, crucial in analyzing the quality, stability, and biological safety of the active pharmaceutical ingredient, Posaconazole. Moreover, Daicel Pharma offers custom synthesis of Posaconazole impurities and delivers them globally.
Posaconazole [CAS: 171228-49-2] is an antifungal drug that belongs to the triazole class. It has asimilar chemical structure to itraconazole and is effective against various types of fungi, includingCryptococcus neoformans, Candida species, andAspergillusspecies.
Posaconazole prevents invasive Aspergillus and Candida infections in high-risk, severely immunocompromised patients. These patients are hematopoietic stem cell transplant recipients with Graft versus Host Disease (GVHD) or have hematologic malignancies with prolonged neutropenia from chemotherapy. It is available under the brand names Noxafil and Noxafil Powdermix Kit.
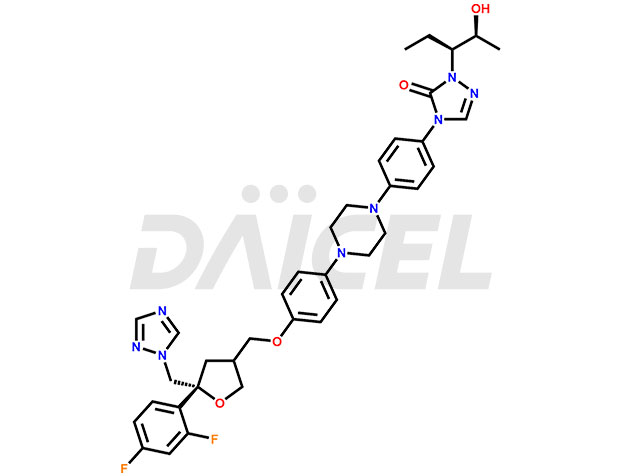
The chemical name of Posaconazole is 2,5-Anhydro-1,3,4-trideoxy-2-C-(2,4-difluorophenyl)-4-[[4-[4-[4-[1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4H-1,2,4-triazol-4-yl]phenyl]-1-piperazinyl]phenoxy]methyl]-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol. It’s chemical formula is C37H42F2N8O4, and its molecular weight is approximately 700.8g/mol.
Posaconazole is an antifungal medication that belongs to the triazole group. It blocks the production of ergosterol, a crucial component of fungal cell membranes, by inhibiting the enzyme lanosterol 14α-demethylase and accumulating methylated sterol precursors.
Posaconazole, an antifungal medication used to treat invasive fungal infections, consists of impurities that can impact its safety and effectiveness during production1 or storage. These impurities include degradation products, intermediates, starting materials, and related substances. Impurity formation is due to poor reaction conditions, unreacted starting materials, or exposure to heat and light during storage. Thus, it is necessary to regularly monitor and control impurities to ensure the safety and quality of Posaconazole.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Posaconazole impurity standards, including Posaconazole Impurity- (component-D), Posaconazole Succinyl Ester, Posaconazole Piperazine dioxide, Posaconazole impurity-B, Posaconazole Impurity 57, Desethylene Posaconazole N-formyl, Desethylene Posaconazole, etc. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We also provide a complete characterization report. Daicel has the technology and expertise to prepare any unknown Posaconazole impurity or degradation product. Daicel also provides labeled compounds to quantify the efficacy of generic Posaconazole. Daicel offers highly pure isotope-labeled standards of Posaconazole for bioanalytical research and BA/BE studies with isotope data in CoA.
Posaconazole impurities originate from various sources, including starting materials, synthetic intermediates, and degradation products.
The common impurities found in Posaconazole include isomers, related compounds, and degradation products.
Isomeric impurities in Posaconazole can affect the potency and bioavailability of the drug.
Prevent the formation of Posaconazole impurities by optimizing the synthetic process, controlling reaction conditions, and using appropriate analytical methods to monitor them.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.