Pirfenidone
General Information
Pirfenidone Impurities and Pirfenidone
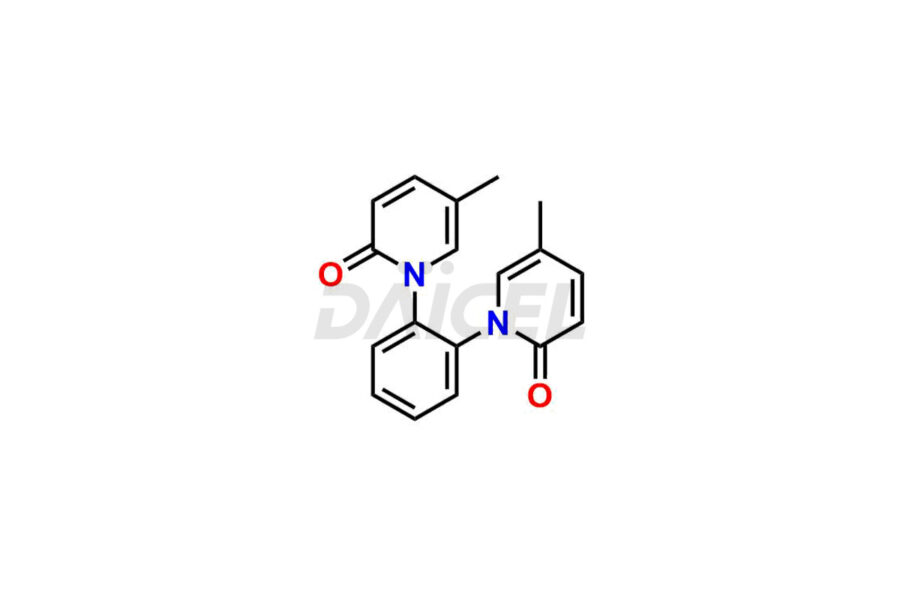
Daicel Pharma offers Pirfenidone impurity standards such as Pirfenidone PIRRC-11, Pirfenidone EP Impurity A, Pirfenidone EP Impurity B, and 2 Hydroxy-5-methylpyridine-N-Oxide. Their presence can affect the effectiveness, stability, and safety of Pirfenidone. Daicel Pharma can synthesize custom Pirfenidone impurities following global standards and regulations while delivering them worldwide.
Pirfenidone [CAS: 53179-13-8] is a pyridinone derivative and an anti-inflammatory medication to treat idiopathic pulmonary fibrosis. It functions as a non-narcotic analgesic, antipyretic, and non-steroidal anti-inflammatory medicine.
Pirfenidone: Use and Commercial Availability
Pirfenidone treats idiopathic pulmonary fibrosis (IPF), a chronic, progressive type of interstitial pneumonia. This drug is available under the tradename of Esbriet.
Pirfenidone Structure and Mechanism of Action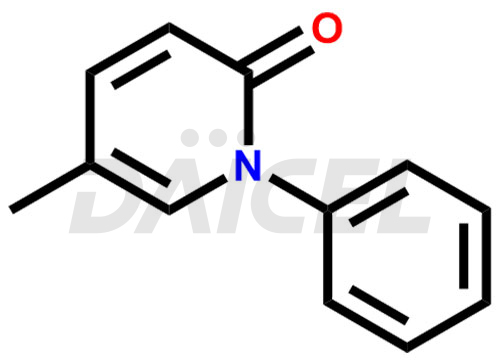
The chemical name of Pirfenidone is 5-Methyl-1-phenyl-2(1H)-pyridinone. Its chemical formula is C12H11NO, and its molecular weight is approximately 185.22 g/mol.
The mechanism of action of Pirfenidone is not known.
Pirfenidone Impurities and Synthesis
Pirfenidone is a drug to treat fibrosis. Impurities may form or be introduced during the production and storage of the drug, affecting its quality, effectiveness, and safety. Pirfenidone synthesis1 includes several processes, and impurities might form at any point. Related chemicals, leftover starting ingredients, reaction byproducts, and degradation products are types of Pirfenidone impurities.
Daicel provides a Certificate of Analysis (CoA) of Pirfenidone impurity standards, including Pirfenidone PIRRC-11, Pirfenidone EP Impurity A, Pirfenidone EP Impurity B, and 2 Hydroxy-5-methylpyridine-N-Oxide. Daicel Pharma, with its analytical facility certified under current Good Manufacturing Practices (cGMP), offers a comprehensive Certificate of Analysis (CoA) that provides detailed characterization information, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, by request, Daicel Pharma can provide further characterization details like 13C-DEPT. We have the technical capabilities to synthesize Pirfenidone impurities and labeled compounds to facilitate the evaluation of generic Pirfenidone. Furthermore, Daicel Pharma offers Pirfenidone-D3, a deuterium-labeled Pirfenidone standard, for bioanalytical studies and Bioavailability/Bioequivalence (BA/BE) research.
References
FAQ's
References
- Gadekar, Shreekrishna M., N-Substituted Pyridone and General Method For Preparing Pyridones, Affiliated Medical Research, Inc., United States, US3839346A, October 1, 1974
- Shi, Shaojun; Wu, Jianhong; Wu, Jun; Zeng, Fandian, Development and Validation of an Improved LC Method for the Simultaneous Determination of Pirfenidone and Its Carboxylic Acid Metabolite in Human Plasma, Chromatographia, Volume: 69, Issue: 5-6, Pages: 459-463, 2009
Frequently Asked Questions
How effectively can Pirfenidone impurities be removed?
Rigorous purification techniques help minimize Pirfenidone impurities significantly and ensure its quality.
What is the significance of analyzing Pirfenidone impurities?
Pirfenidone impurities analysis verifies the drug's purity, safety, and efficacy in treating lung illnesses and fibrosis.
How are Pirfenidone impurities synthesized?
The synthetic process of Pirfenidone impurities involves specific chemical reactions and methods during the manufacturing of the drug.
What are the temperature conditions required to store Pirfenidone impurities?
Pirfenidone impurities should ideally be stored at a controlled room temperature of 2-8°C or as described on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.