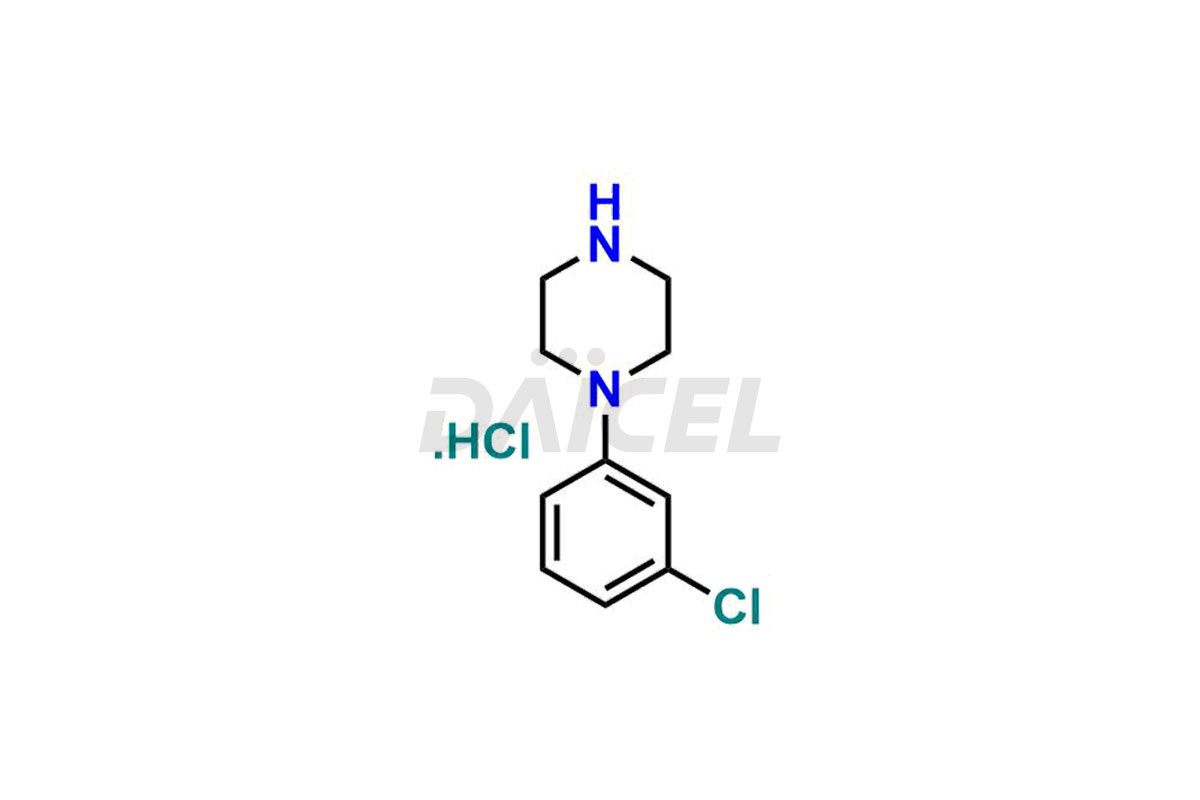
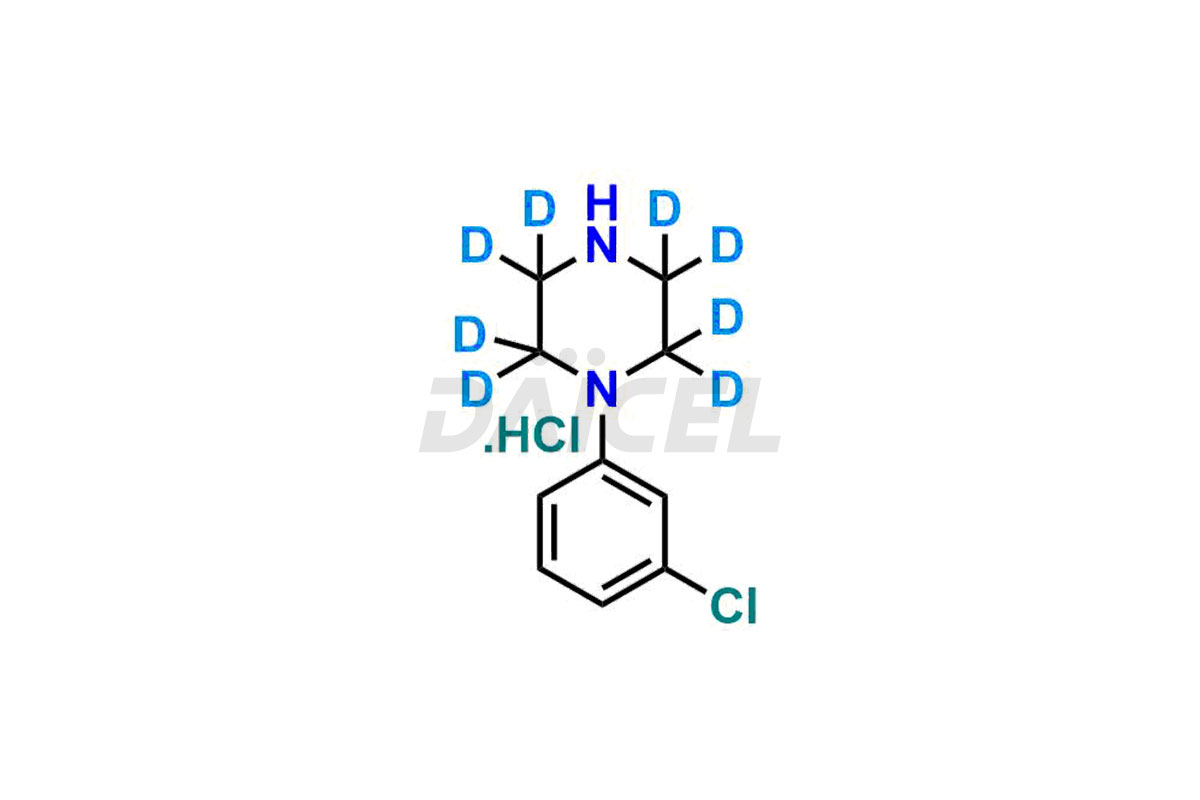
piperazine
General Information
Piperazine Impurities and Piperazine
Daicel Pharma offers high-quality Piperazine impurity standards, which include 1-(3-chlorophenyl)piperazine.HCl. The impurities can influence the efficacy, stability, and safety of Piperazine. Customized Piperazine impurities are produced and distributed by Daicel Pharma worldwide.
Piperazine [CAS: 110-85-0] is a type of azacycloalkane characterized by a six-membered ring that contains two nitrogen atoms positioned across from each other. It also treats parasitic worm infections.
Piperazine: Use and Commercial Availability
Piperazine is an alternate therapy for Ascaris lumbricoides (roundworm) ascariasis and Enterobiasis (oxyuriasis) caused by Enterobius vermicularis (pinworm). It treats partial intestinal blockage caused by the common roundworm, frequent in children. This medication is available in the market under various tradenames such as Antepar, Bryrel, Multifuge, and Vermidol.
Piperazine Structure and Mechanism of Action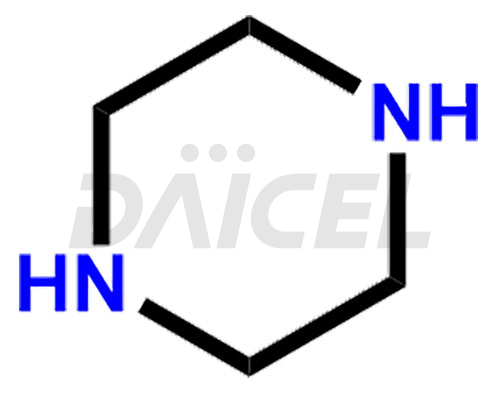
The chemical name of Piperazine is 1,4-Diazacyclohexane. Its chemical formula is C4H10N2, and its molecular weight is approximately 86.14 g/mol.
Piperazine directly binds to muscle membrane GABA receptors and causes hyperpolarization of nerve endings leading to paralysis of worms.
Piperazine Impurities and Synthesis
Piperazine, an organic molecule, may have impurities caused by synthesis1 or storage conditions. They might differ depending on the synthetic technique and the quality of the starting components. They are maintained to a minimum for pure and safe Piperazine.
Daicel Pharma provides a Certificate of Analysis (CoA) for Piperazine impurity standards, which include 1-(3-chlorophenyl)piperazine.HCl. Our cGMP-certified analytical facility gives a comprehensive CoA with detailed characterization data like 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterizations like 13C-DEPT are available upon request. At Daicel Pharma, we synthesize Piperazine impurities and labeled compounds for assessing generic Piperazine and highly pure 1-(3-chlorophenyl)piperazine.HCl-D8, deuterium-labeled Piperazine standards for bioanalytical studies and Bioavailability/Bioequivalence (BA/BE)research.
References
FAQ's
References
- Ishiguro, Takeo; Kitamura, Eiichi; Matsumura, Masaki; Ogawa, Hiroshi, Syntheses of piperazines. V. Syntheses of piperazines from triethanolamine, Yakugaku Zasshi, Volume: 75, Pages: 1367-9, 1955
- Mauger, Anthony B, Gas-liquid chromatography of dioxopiperazines, Journal of Chromatography, Volume: 37, Issue: 2, Pages: 315-17, 1968
Frequently Asked Questions
Can Piperazine impurities be removed?
Piperazine impurities can be removed using proper purifying procedures.
Are there regulatory guidelines for Piperazine impurities?
Piperazine impurities are subject to regulatory oversight.
How are Piperazine impurities synthesized?
Piperazine impurities are synthesized through specific chemical reactions and procedures during the synthetic process.
What are the temperature conditions required to store Piperazine impurities?
Piperazine impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.