LOAD MORE
You're viewed 9 of 50 products
Daicel Pharma is capable of offering Phytonadione impurity standards such as Phytonadione Impurity B, Phytonadione Impurity A, Phytonadione Diol Impurity, Phytonadione Diol Impurity-1 and more. The effectiveness, stability, and safety of Phytonadione is affected by impurities. Daicel Pharma can synthesize custom Phytonadione impurities according to global standards and regulations and worldwide delivery.
Phytonadione [CAS: 84-80-0] belongs to the class of phylloquinone. It functions as a cofactor, as a plant metabolite, and as a human metabolite. It is vitamin K and a phylloquinone member.
Oral phylloquinone treats prothrombin deficiency. It also treats hypoprothrombinemia. Parenteral phylloquinone (administered through intravenous, intramuscular, or subcutaneous routes) treats coagulation disorders caused by deficiencies in the production of coagulation factors II, VII, IX, and X due to vitamin K deficiency or any interference with vitamin K activity. This medicine is available under brand names such as Aquamephyton, Konakion, Mephyton, Vitamin K1, and Vitaped.
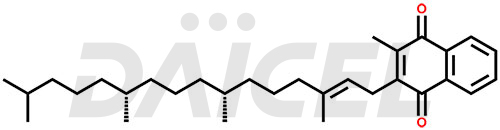
The chemical name of Phytonadione is 2-Methyl-3-[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]-1,4-naphthalenedione. Its chemical formula is C31H46O2, and its molecular weight is approximately 450.7 g/mol.
Phytonadione is necessary for the formation of prothrombinogen and other blood clotting factors in the liver.
Phytonadione impurities refer to unintended substances or contaminants present in Phytonadione1, a synthetic form of vitamin K. These impurities can impact the product’s effectiveness, stability, and safety.
Daicel Pharma provides a Certificate of Analysis (CoA) for various Phytonadione impurity standards, including Phytonadione Impurity B, Phytonadione Impurity A, Phytonadione Diol Impurity, Phytonadione Diol Impurity-1, and more. Our analytical facility, certified under cGMP, issues a comprehensive CoA and detailed characterization information such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization details, like 13C-DEPT, can be provided upon request. Daicel Pharma is qualified to provide Phytonadione impurities and labeled compounds for assessing the effectiveness of generic Phytonadione. We offer highly pure Cis-Phytonadione-D7, Phytonadione-D7 (Mixture of Cis & Trans), and Tran-Phytonadione-D7, deuterium-labeled Phytonadione standard, essential for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
Complete removal of Phytonadione impurities is achievable through appropriate purification processes and quality control measures.
Yes, there are regulatory guidelines in place for Phytonadione impurities.
Pharmaceutical manufacturers take several measures to minimize impurities in Phytonadione. These include employing Good Manufacturing Practices (GMP), using high-quality raw materials, optimizing preparing processes, implementing proper storage and handling procedures, and conducting regular testing and monitoring to ensure product quality and purity.
Phytonadione impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.