Phenylephrine
General Information
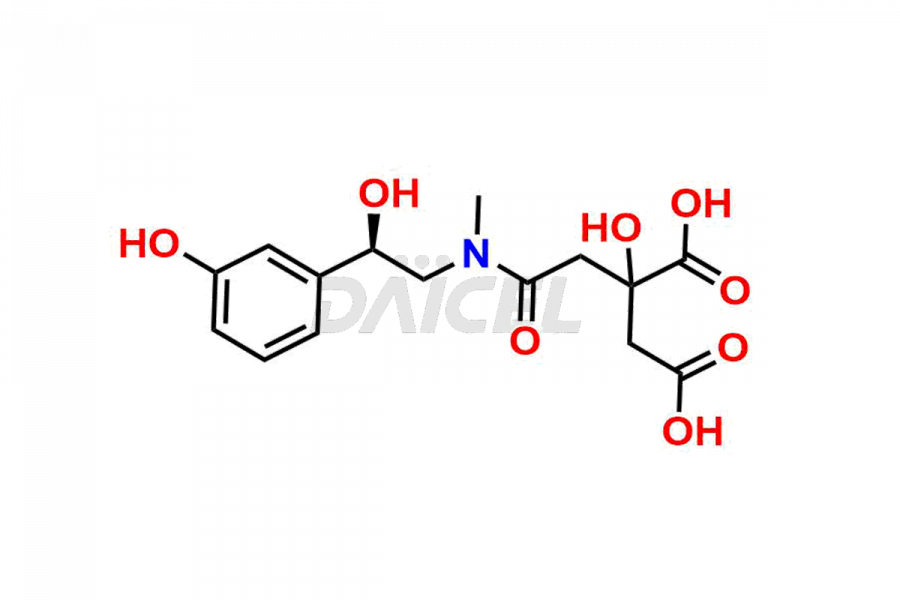
Phenylephrine Impurities and Phenylephrine
Daicel Pharma offers high-quality Phenylephrine impurity standards, Phenyl Ephrine D-(+)-Glucose Adduct, Phenylephrine citrate adduct, (R)-4-chloro-3-(1-hydroxy-2-(methylamino)ethyl)phenol hydrochloride, and Phenylephrine Impurity 30. The impurities can affect the effectiveness, stability, and safety of Phenylephrine. Daicel Pharma specializes in synthesizing custom Phenylephrine impurities in compliance with international standards and regulations.
Phenylephrine [CAS: 59-42-7] is a phenylethanolamine that acts as an alpha-adrenergic agonist and a cardiotonic drug. It also acts as a mydriatic agent, a protective agent, a vasoconstrictor agent, a sympathomimetic agent, and a nasal decongestant.
Phenylephrine: Use and Commercial Availability
Alpha-1 adrenergic receptor agonist, Phenylephrine, treats hypotension due to shock or anesthesia. It also treats congestion. Topical preparations treat hemorrhoids. This medication is available under brand names such as Biorphen, Immphentiv, Vazculep, etc.
Phenylephrine Structure and Mechanism of Action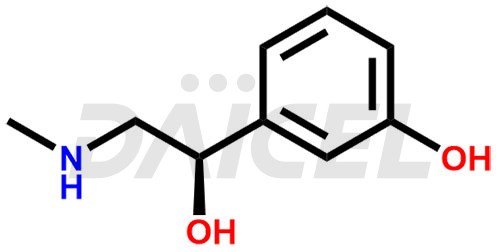
The chemical name of Phenylephrine is (αR)-3-Hydroxy-α-[(methylamino)methyl]benzenemethanol. Its chemical formula is C9H13NO2, and its molecular weight is approximately 167.21 g/mol.
Phenylephrine interacts with a-1 adrenergic receptors, causes activation of smooth muscle cells, and leads to vasoconstriction.
Phenylephrine Impurities and Synthesis
Impurities in Phenylephrine can be generated during its synthesis1 through different routes, resulting from incomplete reactions, side reactions, and contaminants in the starting materials and reagents. The synthetic pathways for Phenylephrine impurities can differ based on the particular impurity level and the specific conditions employed during the process.
Daicel Pharma provides a Certificate of Analysis (CoA) for Phenylephrine impurity standards that include Phenyl Ephrine D-(+)-Glucose Adduct, Phenylephrine citrate adduct, (R)-4-chloro-3-(1-hydroxy-2-(methylamino)ethyl)phenol hydrochloride, and Phenylephrine Impurity 30. Our cGMP-certified analytical facility at Daicel Pharma issues a Certificate of Analysis (CoA) that contains detailed characterization information, 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, further characterization details, including 13C-DEPT, can be given. Daicel Pharma provides Phenylephrine impurities and degradation products.
References
FAQ's
References
- Legerlotz, H., A process for preparing m- and p-hydroxy-phenyl-n-methyl-amino-ethanol-1, GB354226A, July 27, 1931
- Montgomery, Kenneth O.; Jennings, P. V.; Weinswig, Melvin H., Ion-exchange separation and ultraviolet determination of phenylephrine, codeine, and selected antihistamines, Journal of Pharmaceutical Sciences, Volume: 56, Issue: 1, Pages: 141-3, 1967
Frequently Asked Questions
Can Phenylephrine impurities be removed?
Depending on the impurity and its concentration, purification techniques such as recrystallization, column chromatography, or other purification methods can be employed to remove or reduce them in Phenylephrine.
Are there regulatory guidelines for Phenylephrine impurities?
Regulatory authorities provide guidelines and limits for impurities in pharmaceutical substances, including Phenylephrine, to ensure product quality and safety.
What are the temperature conditions required to store Phenylephrine impurities?
Phenylephrine impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Why are impurities a concern in Phenylephrine?
Impurities in Phenylephrine can have potential safety and efficacy implications. Some may be toxic or have undesirable effects on the body. They can also affect the stability and quality of the drug, potentially reducing its effectiveness or causing adverse reactions in patients.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.