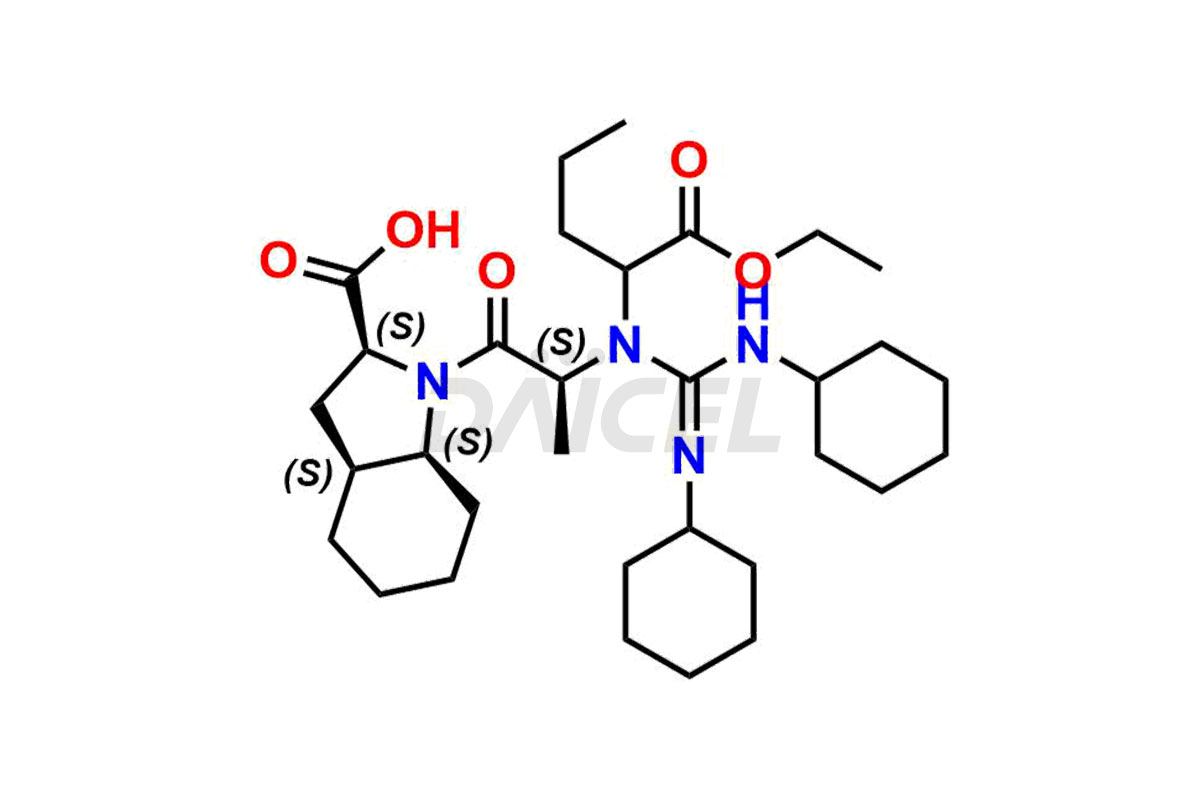
Perindopril
References
- Vincent, M.; Remond, G.; Portevin, B.; Serkiz, B.; Laubie, M., Stereoselective synthesis of a new perhydroindole derivative of chiral iminodiacid, a potent inhibitor of angiotensin converting enzyme, Tetrahedron Letters, Volume: 23, Issue: 16, Pages: 1677-80, 1982
- Lin, S. J.; Wu, H. L.; Chen, S. H.; Wen, Y. H., Derivatization-gas chromatographic determination of perindopril, Analytical Letters, Volume: 29, Issue: 10, Pages: 1751-1762, 1996
Frequently Asked Questions
How are Perindopril impurities formed?
Impurities in Perindopril can form through various routes, including degradation reactions during synthesis, storage, or exposure to environmental factors, as well as impurity introduction from raw materials or by-products of the synthesis process.
What is the significance of analyzing Perindopril impurities?
Analyzing Perindopril impurities is significant for ensuring safety, efficacy, and quality, as they may affect its effectiveness, stability, and overall safety profile.
How are Perindopril impurities identified and classified?
Following regulatory rules and standards, Perindopril impurities are discovered and categorized using analytical techniques, including chromatography, spectroscopy, and mass spectrometry, based on their chemical structure, retention duration, and spectral data.
What are the temperature conditions required to store Perindopril impurities?
Perindopril impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.