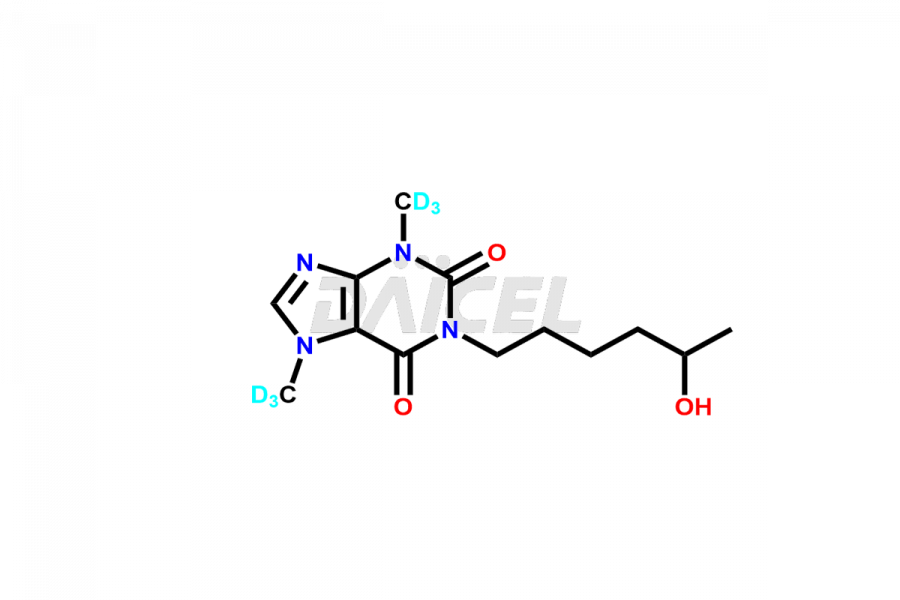
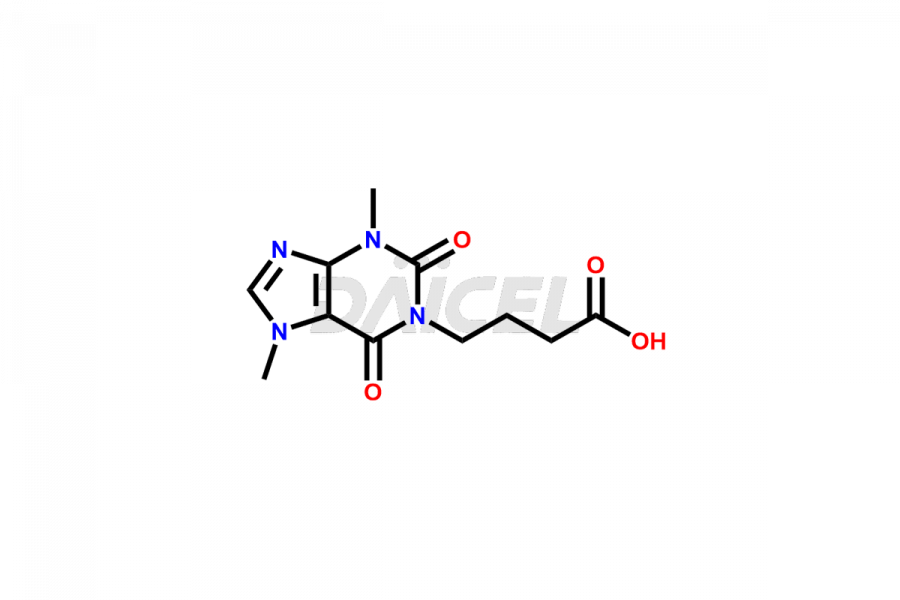
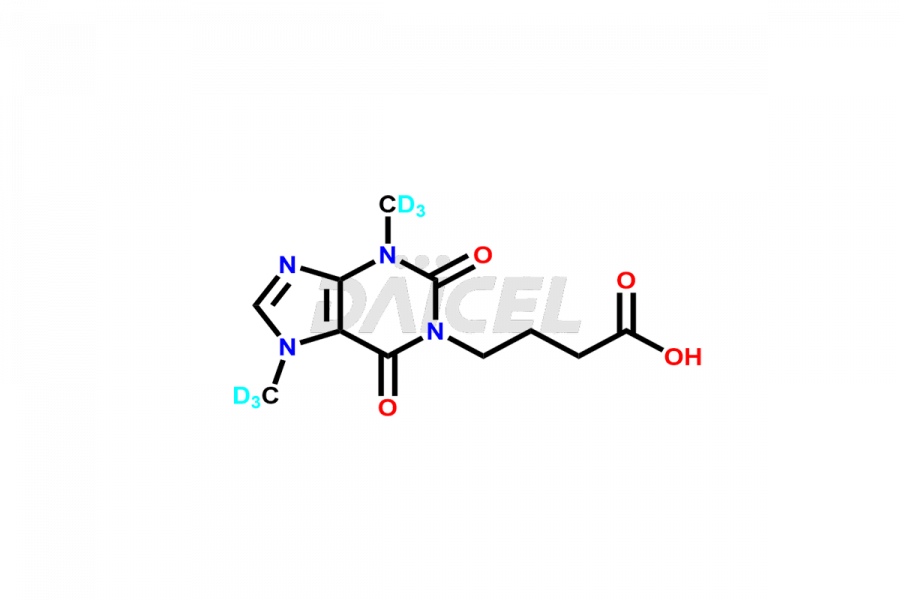
Pentoxifylline
General Information
Pentoxifylline Impurities and Pentoxifylline
Daicel Pharma offers Pentoxifylline impurity standards like Pentoxifylline Metabolite V and Pentoxifylline Metabolite I. The impurities can impact Pentoxifylline efficacy, stability, and safety. Daicel Pharma can synthesize Pentoxifylline impurities and deliver them worldwide.
Pentoxifylline [CAS: 6493-05-6] is a synthetic compound derived from dimethylxanthine that modifies blood flow while possessing both anti-oxidant and anti-inflammatory properties. It treats symptoms of intermittent claudication due to peripheral arterial disease.
Pentoxifylline: Use and Commercial Availability
Pentoxifylline treats patients with chronic occlusive artery disease who have intermittent claudication. It may enhance limb function and lessen discomfort but cannot replace other treatments, such as surgical bypass surgery. Pentoxifylline, a xanthine derivative, reduces blood viscosity. This medication is available under certain tradenames such as Pentoxil and Trental.
Pentoxifylline Structure and Mechanism of Action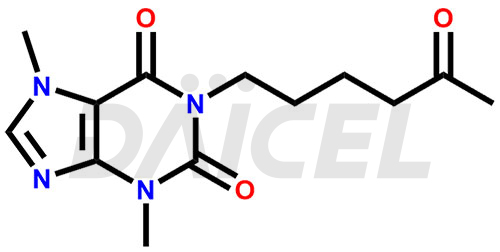
The chemical name of Pentoxifylline is 3,7-Dihydro-3,7-dimethyl-1-(5-oxohexyl)-1H-purine-2,6-dione. Its chemical formula is C13H18N4O3, and its molecular weight is approximately 278.31 g/mol.
Pentoxifylline decreases the viscosity of blood and improves blood flow properties.
Pentoxifylline Impurities and Synthesis
Impurities in Pentoxifylline can develop at several stages of the synthetic process1, such as incomplete reactions, side reactions, and contaminants in the raw materials and reagents. Depending on the individual impurity and the circumstances, different Pentoxifylline impurities can synthesize using methods.
Daicel Pharma offers a Certificate of Analysis (CoA) for Pentoxifylline impurity standards, such as Pentoxifylline Metabolite V and Pentoxifylline Metabolite I. We provide a Certificate of Analysis (CoA) from our cGMP-certified analytical lab, which includes extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization information, including 13C-DEPT, can be provided upon request. Our team at Daicel Pharma specializes in offering Pentoxifylline impurities and degradation products. We offer Pentoxifylline Metabolite V-d6, Pentoxifylline Metabolite I-D6, 7-(Methyl-D3)-Xanthine, and 1,3,7- Trimethylxanthine-d3, highly pure deuterium-labeled Pentoxifylline compounds, valuable for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) researches.
References
FAQ's
References
- Chemische Werke Albert, Xanthine derivatives, GB1079267A, August 16, 1967
- Chivers, D. A.; Birkett, D. J.; Miners, J. O, Simultaneous determination of pentoxifylline and its hydroxy metabolite in plasma by high-performance liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 225, Issue: 1, Pages: 261-5, 1981
Frequently Asked Questions
Can Pentoxifylline impurities be removed?
Pentoxifylline impurities are reduced but not removed.
What is the significance of analyzing Pentoxifylline impurities?
Analyzing Pentoxifylline impurities is significant for ensuring safety, efficacy, and quality, as it can impact its effectiveness and pose potential risks to patients.
How are Pentoxifylline impurities synthesized?
Pentoxifylline impurities are synthesized through various pathways during the synthetic process, including incomplete reactions, side reactions, and their presence in starting materials or reagents.
What are the temperature conditions required to store Pentoxifylline impurities?
Pentoxifylline impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.