Pelubiprofen
General Information
Pelubiprofen Impurities and Pelubiprofen
Daicel Pharma offers high-quality Pelubiprofen impurity standards, Pelubiprofen Cis-OH, and Pelubiprofen Trans-OH. The presence of these impurities can impact the efficacy, stability, and safety of Pelubiprofen. Daicel Pharma can synthesize custom Pelubiprofen impurities and offer them under worldwide standards and needs.
Pelubiprofen [CAS: 69956-77-0], a commercialized non-steroidal anti-inflammatory medication (NSAID), has been investigated for treating chronic back pain.
Pelubiprofen: Use and Commercial Availability
Pelubiprofen is an effective non-steroidal anti-inflammatory drug (NSAID) that alleviates pain and inflammation related to various musculoskeletal conditions. It is for disorders, including osteoarthritis, post-operative trauma, backache, neck-shoulder syndrome, dental pain, and rheumatoid arthritis.
Pelubiprofen Structure and Mechanism of Action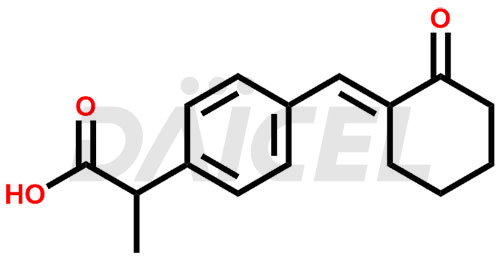
The chemical name of Pelubiprofen is α-Methyl-4-[(E)-(2-oxocyclohexylidene)methyl]benzeneacetic acid. Its chemical formula is C16H18O3, and its molecular weight is approximately 258.31 g/mol.
Pelubiprofen decreases prostaglandin synthesis by inhibiting cyclooxygenases (COXs) and IkB kinase-b (IKK-b) activities.
Pelubiprofen Impurities and Synthesis
Impurities can occur during the synthesis1 of Pelubiprofen by incomplete reactions, side reactions, contaminants in the starting materials, or reagents utilized. Strict quality control measures are needed while preparing Pelubiprofen to avoid unwanted side effects caused by these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Pelubiprofen impurity standards, Pelubiprofen Cis-OH, and Pelubiprofen Trans-OH. We offer a Certificate of Analysis (CoA) from our cGMP-certified analytical laboratory, which contains comprehensive characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additional characterization details, such as 13C-DEPT, can be provided upon request. At Daicel Pharma, our experts specialize in synthesizing Pelubiprofen impurities and degradation products.
References
FAQ's
References
- Terada, Atsusuke; Tanaka, Shigeru; Misaka, Eiichi, Cycloalkylidenemethylphenylacetic acid derivatives and process for the preparation thereof, Sankyo Co., Ltd., Japan, US4254274A, March 3, 1981
- Ryu, Ju-Hee; Park, Ji-Sun; Jo, Min-ho; Kim, Joo-Il; Shim, Wang-Seob; Kim, Bo-Hyung; Yim, Sung-Vin; Hong, Jongki; Lee, Kyung-Tae, Development and validation of an LC-MS/MS method for the determination of pelubiprofen and its active metabolite, trans-alcohol, in human plasma and its application to pharmacokinetic study, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 983-984, Pages: 62-67, 2015
Frequently Asked Questions
Are there any Pelubiprofen impurities concern in specific patient populations or conditions?
No impurities in Pelubiprofen are known to be of concern in patient-specific populations or conditions.
What is the significance of analyzing Pelubiprofen impurities?
Pelubiprofen impurities must be analyzed to guarantee the medication's safety, efficacy, and quality and to assure compliance with regulatory rules and specifications.
How do Pelubiprofen impurities form?
Pelubiprofen impurities form during the preparation due to incomplete reactions, side reactions, and contaminants in starting materials or reagents.
What are the temperature conditions required to store Pelubiprofen impurities?
Pelubiprofen impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.



