LOAD MORE
You're viewed 9 of 15 products
Daicel Pharma offers Pazopanib impurity standards, including Pazopanib Dimer, Pazopanib Impurity 9, Pazopanib impurity 8, Pazopanib N-oxide (RRT-0.70), Pazopanib Pyrimidine impurity and more. The impurities can impact the efficacy, stability, and safety of Pazopanib. Daicel Pharma can synthesize custom Pazopanib impurities and delivers them worldwide.
Pazopanib [CAS: 444731-52-6] is a pyrimidine derivative treating kidney cancer. Pazopanib acts as an antineoplastic agent, a tyrosine kinase inhibitor, a vascular endothelial growth factor receptor antagonist, and an angiogenesis-modulating agent.
Pazopanib acts as an antineoplastic agent and a tyrosine kinase inhibitor, targeting vascular endothelial growth factor receptors and influencing angiogenesis. It treats patients with advanced renal cell carcinoma (RCC). Classified as an indazole, Pazopanib possesses a distinct chemical structure featuring a substituted pyrimidine and a sulfonamide group. It is primarily administered in its hydrochloride salt form to leverage its therapeutic effects. This medication is available under the trade name Votrient.
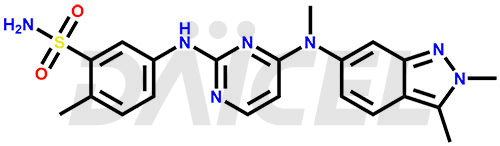
The chemical name of Pazopanib is 5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide. Its chemical formula is C21H23N7O2S, and its molecular weight is approximately 437.5 g/mol.
As an oral small-molecule multi-kinase inhibitor, Pazopanib predominantly inhibits vascular endothelial growth factor receptors 1, 2, and 3, platelet endothelial growth factor receptors 1, 2, and 3, and the cytokine receptor kit.
Impurities can occur during the synthesis1 of Pazopanib by mechanisms, including incomplete reactions, side reactions, and the contaminants in the starting materials or reagents. Strict quality control measures are needed while preparing Pazopanib to avoid unwanted side effects caused by these impurities.
Daicel Pharma offers a Certificate of Analysis (CoA) for Pazopanib impurity standards that involve Pazopanib Dimer, Pazopanib Impurity 9, Pazopanib impurity 8, Pazopanib N-oxide (RRT-0.70), Pazopanib Pyrimidine impurity and more. Our CoA is from our cGMP-certified analytical laboratory and includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. More characterization details, such as those for 13C-DEPT, can be provided on request. Our team of professionals at Daicel Pharma specializes in providing Pazopanib impurities and degradation compounds for testing the efficacy of generic Pazopanib. We provide Pazopanib-d3, a deuterium-labeled Pazopanib standard essential for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
The specific degradation products of Pazopanib impurities can vary, but common degradation pathways may involve oxidative processes, hydrolysis, or photodegradation, leading to various byproducts and unreacted compounds.
Pazopanib impurities are typically tested for mutagenicity to evaluate their potential.
Potential isomeric forms or trace-level impurities of Pazopanib can be more challenging to identify and control due to their low concentration or structural similarities with the main compound.
Pazopanib impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.