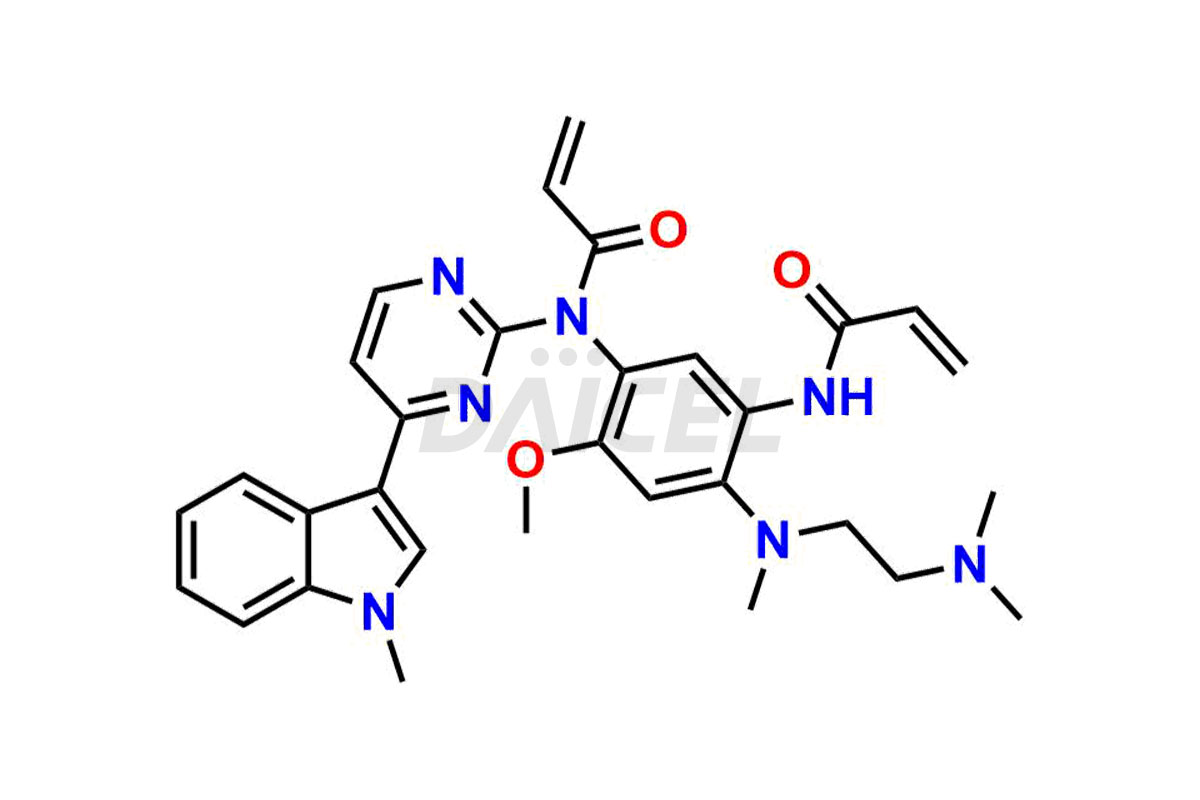
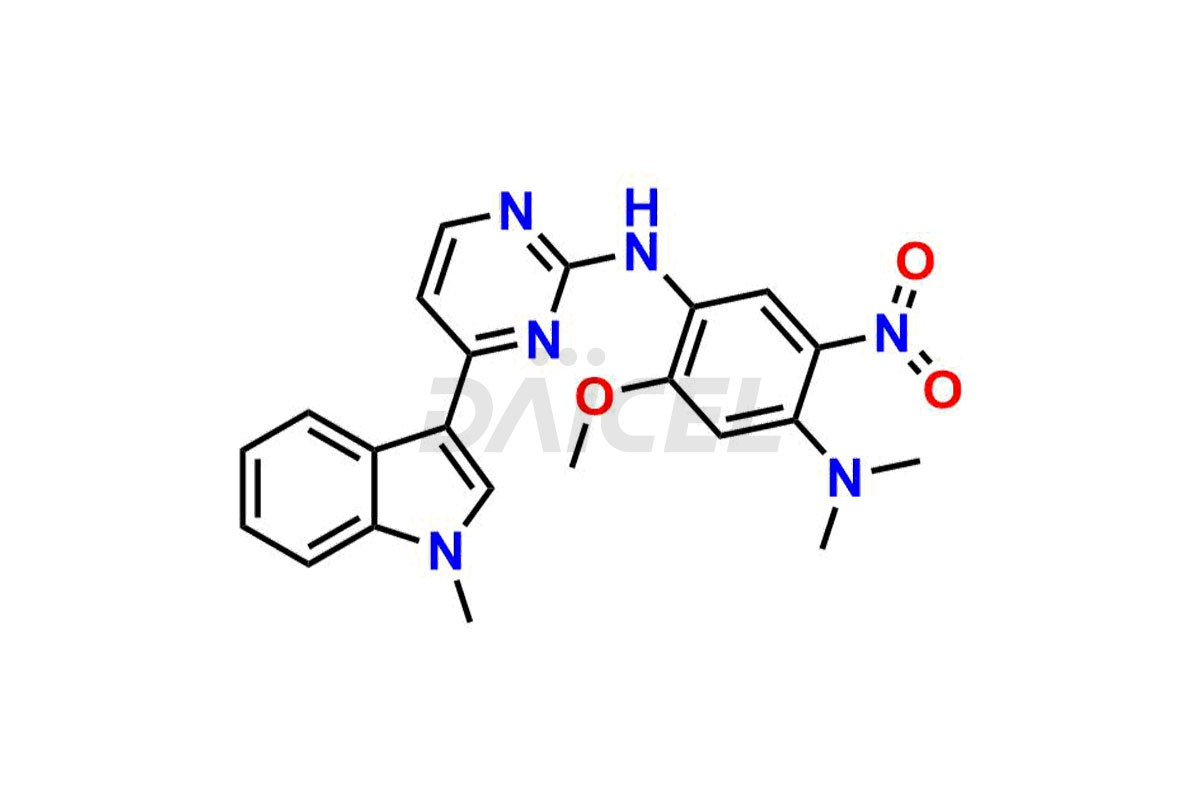
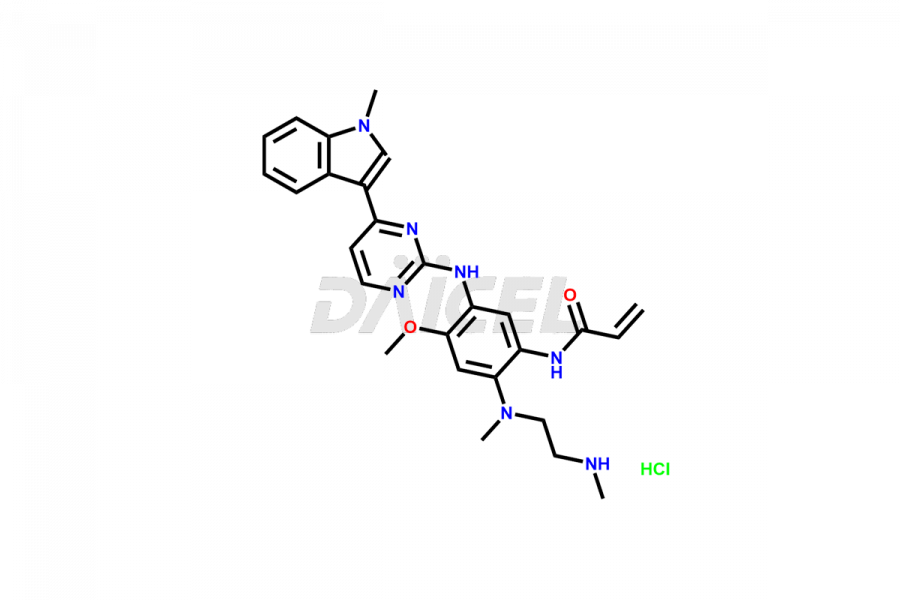
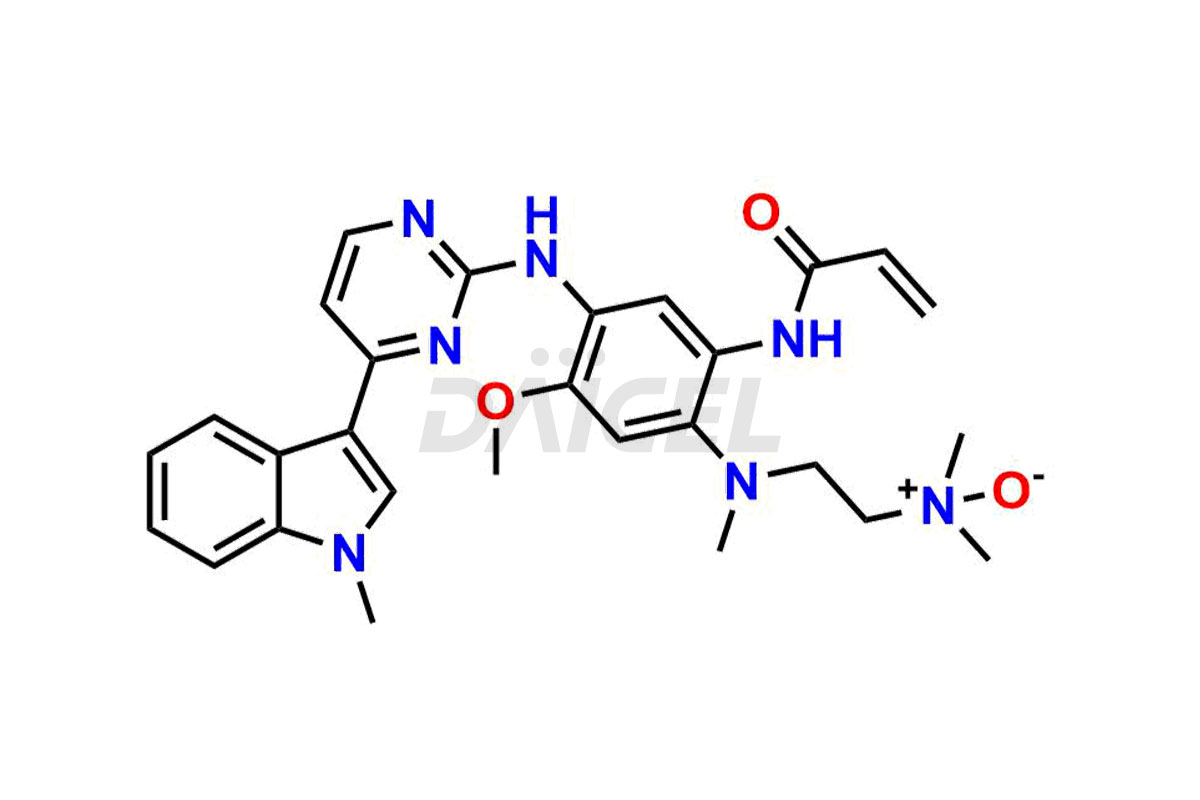
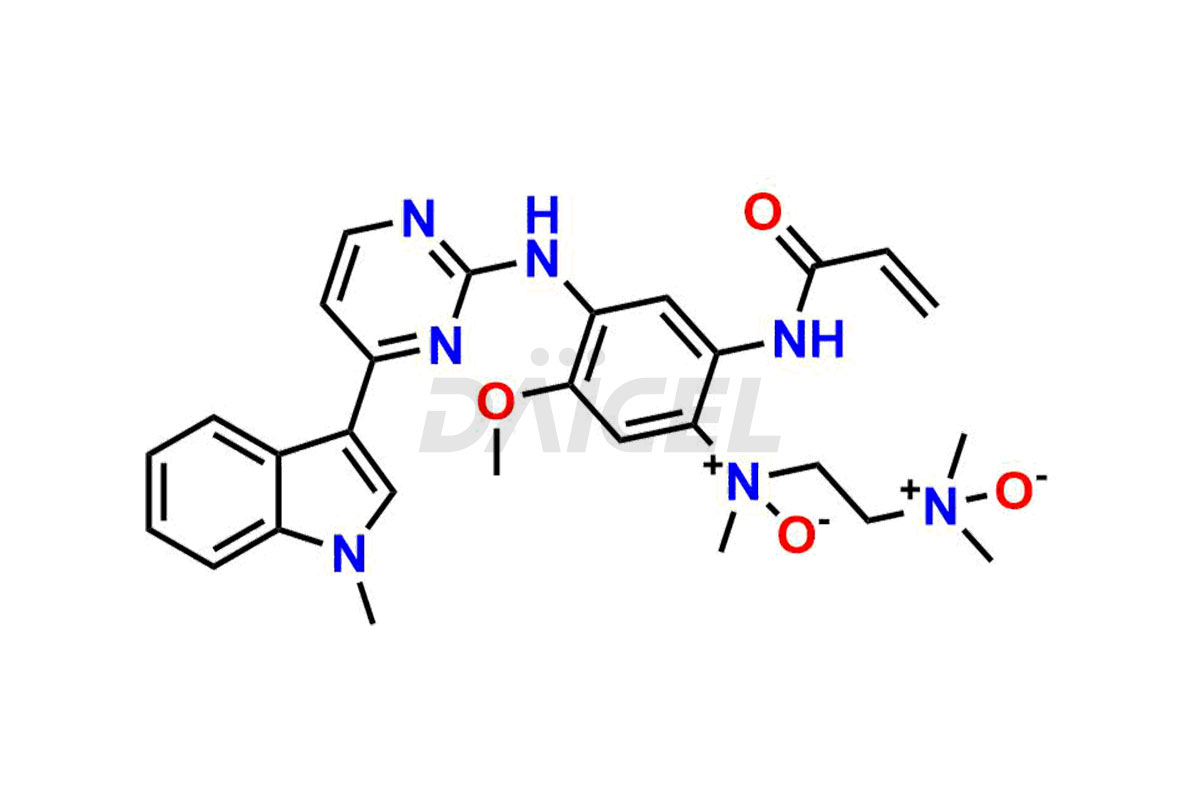
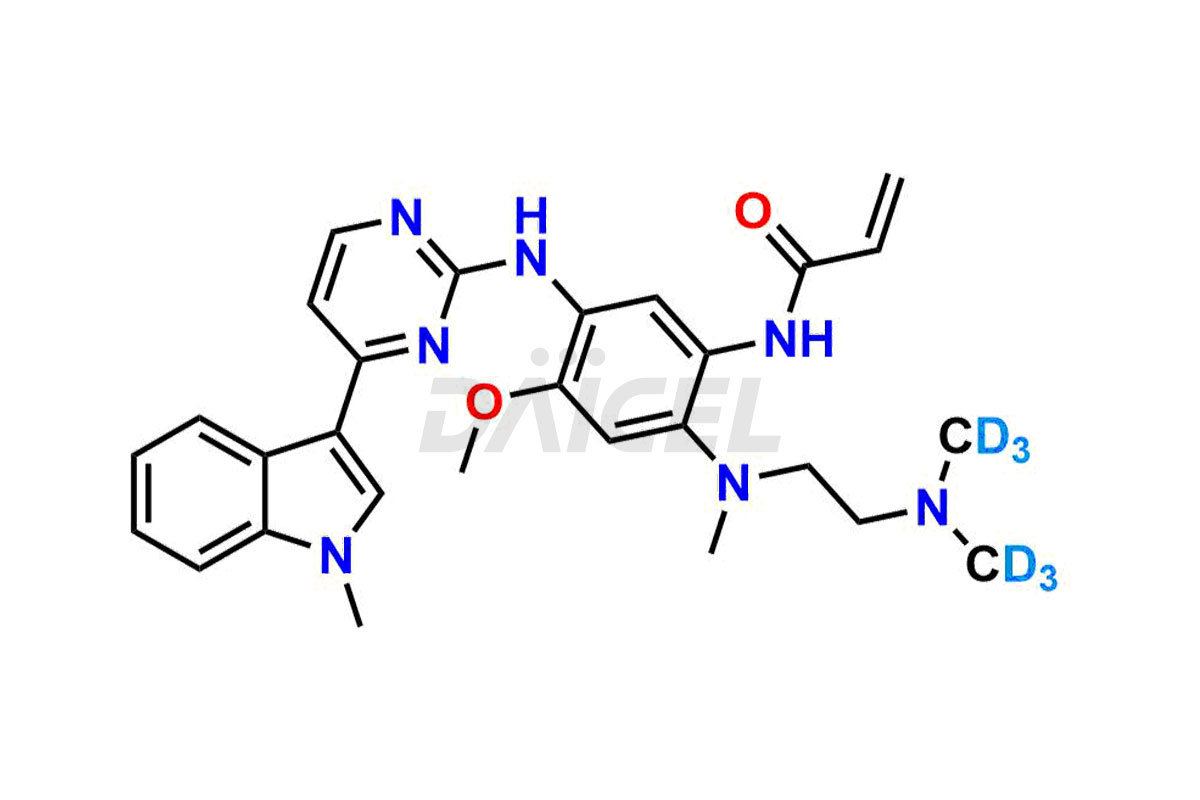
Osimertinib
General Information
Osimertinib Impurities and Osimertinib
Daicel Pharma synthesizes high-quality Osimertinib impurities such as Osimertinib Impurity N, Osimertinib N desmethyl impurity, Osimertinib N-Oxide, Osimertinib N, N’-Dioxide, Osimertinib Impurity-J, and Osimertinib Impurity-I, crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient, Osimertinib. Moreover, Daicel Pharma offers custom synthesis of Osimertinib impurities and delivers them globally.
Osimertinib [CAS: 1421373-65-0] is a small molecule, tyrosine kinase receptor inhibitor, and antineoplastic agent. It treats certain forms of advanced non-small cell lung cancer (NSCLC).
Osimertinib: Use and Commercial Availability
Osimertinib is an oral medication sold under the brand name Tagrisso. It treats adults with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive NSCLC that has progressed on or after EGFR TKI therapy. Further, Tagrisso is the first treatment for patients having ‘activating mutations’ whose cancer has advanced or spread.
Osimertinib Structure and Mechanism of Action 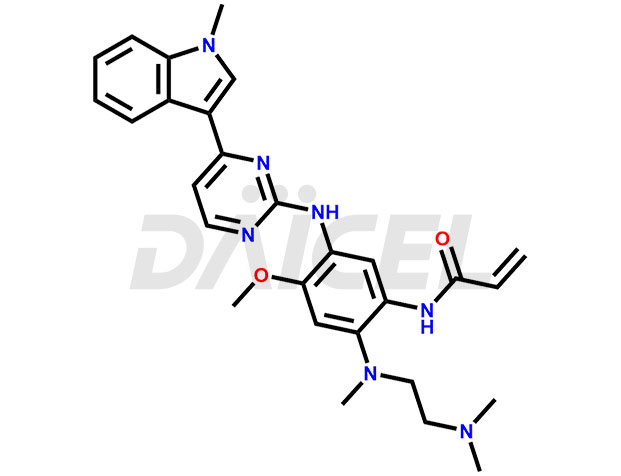
The chemical name of Osimertinib is N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide. Its formula for Osimertinib is C28H33N7O2, and its molecular weight is approximately 499.6 g/mol.
Osimertinib is an EGFR kinase inhibitor, and it irreversibly binds to certain mutant forms of EGFR (T790M, L858R, exon 19 deletion). It binds at approximately 9-fold lower concentrations than wild-type EGFR.
Osimertinib Impurities and Synthesis
Osimertinib may contain related substances and process impurities that impact its final purity, yield, and quality. It is crucial to regulate impurity levels in Osimertinib to maintain the drug’s safety and effectiveness.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Osimertinib impurity standards, including Osimertinib Impurity N, Osimertinib N-desmethyl impurity, Osimertinib N-Oxide, Osimertinib N, N’-Dioxide, Osimertinib Impurity-J, and Osimertinib Impurity-I. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS1, and HPLC purity2. We also provide 13C-DEPT and CHN on request. We also give a complete characterization report on delivery.
Daicel has the technology and expertise to prepare any unknown Osimertinib impurity or degradation product. Daicel also provides labeled compounds to quantify the efficacy of generic Osimertinib. Daicel offers Osimertinib-D6, a deuterium-labeled standard of Osimertinib for bioanalytical research and BA/BE studies.
Osimertinib
References
FAQ's
References
- Rood, Johannes J. M. ; van Bussel, Mark T. J.; Schellens, Jan H. M.; Beijnen, Jos H.; Sparidans, Rolf W.,” Liquid chromatography-tandem mass spectrometric assay for the T790M mutant EGFR inhibitor osimertinib (AZD9291) in human plasma”, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 1031, Pages: 80-85, 2016
- Rood, J. J. M.; van Haren, M. J.; Beijnen, J. H.; Sparidans, R. W., “Bioanalysis of EGFRm inhibitor osimertinib, and its glutathione cycle- and desmethyl metabolites by liquid chromatography-tandem mass spectrometry”, Journal of Pharmaceutical and Biomedical Analysis, Volume: 177, Pages: 112871, 2020
- Butterworth, Sam; Finlay, Maurice Raymond Verschoyle; Ward, Richard Andrew; Kadambar, Vasantha Krishna; Chandrashekar, Reddy C.; Murugan, Andiappan; Redfearn, Heather Marie, “2-(2,4,5-substituted-anilino) pyrimidine compounds”, AstraZeneca AB, Sweden, US8946235B2, Feb 3, 2015
Frequently Asked Questions
What are the most common Osimertinib impurities?
The most common impurities in Osimertinib are N-desmethyl Osimertinib, Osimertinib N-oxide, Osimertinib oxide, and Osimertinib desmethoxy.
What impact do Osimertinib impurities have on the stability of the drug?
Impurities in Osimertinib can potentially affect its stability, leading to drug degradation over time. It could affect the safety, efficacy, and shelf life of the drug.
How are Osimertinib impurities detected?
Impurities in Osimertinib detection are through analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography mass spectrometry (LC-MS), etc.
How are Osimertinib impurities removed?
Osimertinib impurities removal is by purification steps, such as crystallization or chromatography.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.