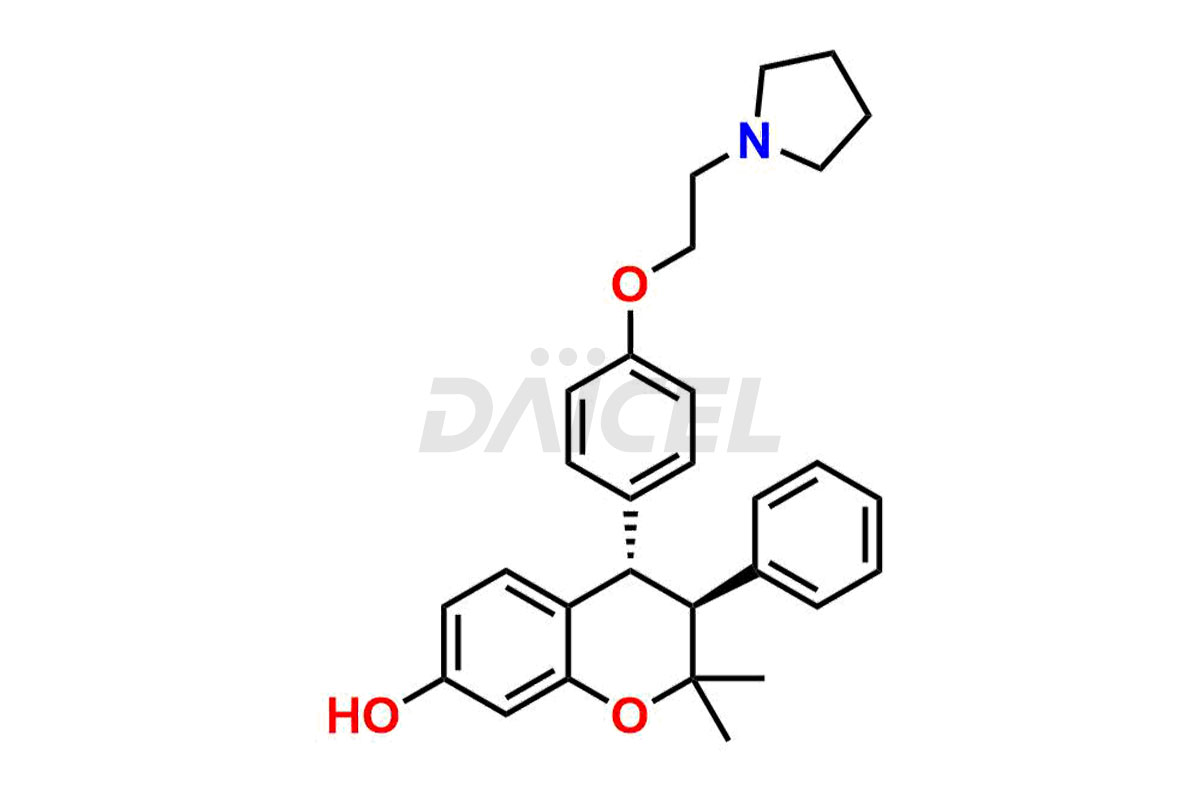
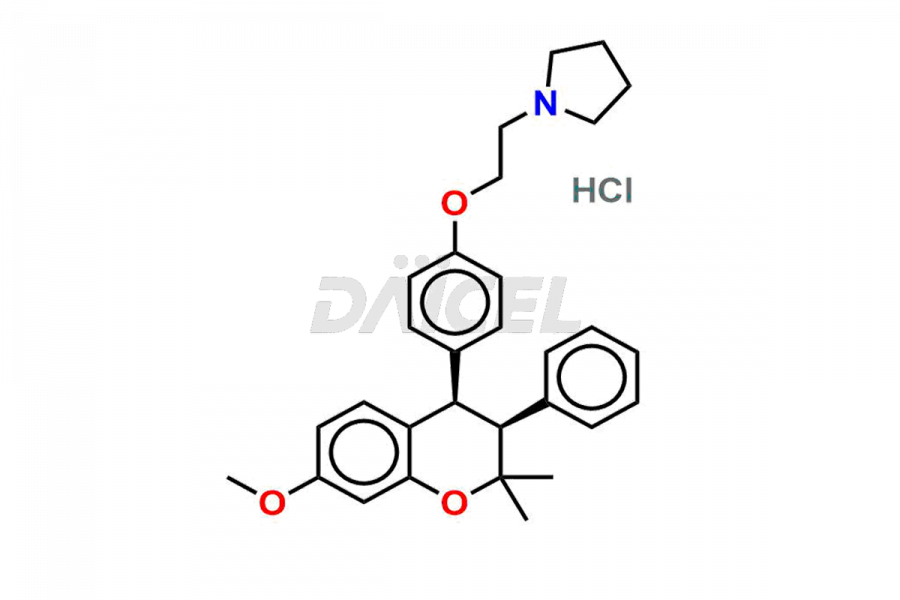
Ormeloxifene
General Information
Ormeloxifene Impurities and Ormeloxifene
Daicel Pharma offers Ormeloxifene Impurity standards, including 7-Desmethyl Impurity and CIS CENTCHROMAN HCl. They determine the quality, stability, and safety of Ormeloxifene, an active pharmaceutical ingredient (API). Daicel Pharma can synthesize custom Ormeloxifene impurities and distribute them across the world.
Ormeloxifene [CAS: 31477-60-8] is a nonsteroidal contraceptive medication for selective antiestrogen that
manages dysfunctional uterine bleeding.
Ormeloxifene: Use and Commercial Availability
Ormeloxifene is a nonsteroidal medication that selectively inhibits estrogen. It effectively blocks estrogen and treats mastalgia and many tiny fibroadenomas. It is available under the tradename Centchroman.
Ormeloxifen Structure and Mechanism of Action 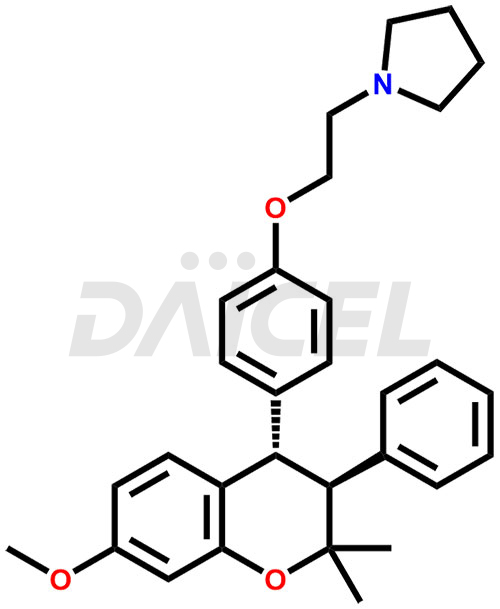
The chemical name of Ormeloxifene is rel-1-[2-[4-[(3R,4R)-3,4-Dihydro-7-methoxy-2,2-dimethyl-3-phenyl-2H-1-benzopyran-4-yl]phenoxy]ethyl]pyrrolidine. Its chemical formula is C30H35NO3, and its molecular weight is approximately 459.6 g/mol.
Ormeloxifene prevents embryo implantation and endometrial receptivity.
Ormeloxifene Impurities and Synthesis
Ormeloxifene is a selective estrogen receptor modulator (SERM) used as a contraceptive for treating abnormal uterine bleeding. During the synthesis1 of Ormeloxifene, impurities form through various processes. They may arise from incomplete reactions, side reactions, or contaminants in the starting materials or reagents. Additionally, impurities can occur during the storage or degradation of Ormeloxifene.
Daicel Pharma offers a Certificate of Analysis (CoA) for Ormeloxifene impurity standards, including the 7-Desmethyl Impurity and CIS CENTCHROMAN HCl. Our CoA is from a cGMP-certified analytical facility and contains comprehensive characterization information such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. Additional characterization information, including 13C-DEPT, can be provided upon request. At Daicel Pharma, our team of experts specializes in synthesizing Ormeloxifene impurities.
References
FAQ's
References
- Bolger, James W., Process For Preparation Of Substituted 3,4-(Diphenyl)Chromans, Rexall Drug and Chemical Co., US3822287A, July 2, 1974
- Shivaraj, Anusha; Battula, Shireesha, Method development and validation of RP-HPLC method for the estimation of Ormeloxifene, International Journal of Applied Pharmaceutical Sciences and Research, Volume: 2, Issue: 3, Pages: 78-82, Journal, 2017
Frequently Asked Questions
Can Ormeloxifene impurities be removed?
It is challenging to remove all impurities in Ormeloxifene. However, measures help minimize impurity levels during the preparation process and ensure they are within acceptable limits defined by regulatory authorities.
What is the significance of analyzing Ormeloxifene impurities?
Analyzing Ormeloxifene impurities is significant to ensure the safety, efficacy, and quality of the medication by identifying, quantifying, and controlling unwanted substances that may have toxic or adverse effects on patients or interfere with the desired therapeutic action of Ormeloxifene.
How are Ormeloxifene impurities synthesized?
Ormeloxifene impurities synthesize through various mechanisms during preparation, including incomplete reactions, side reactions, and their presence in starting materials or reagents.
What are the temperature conditions required to store Ormeloxifene impurities?
Ormeloxifene impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.