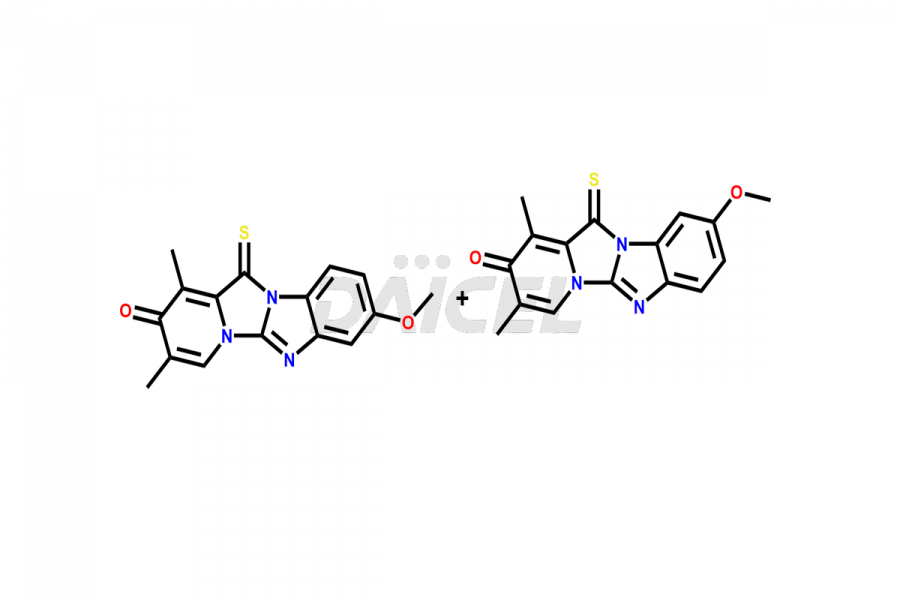
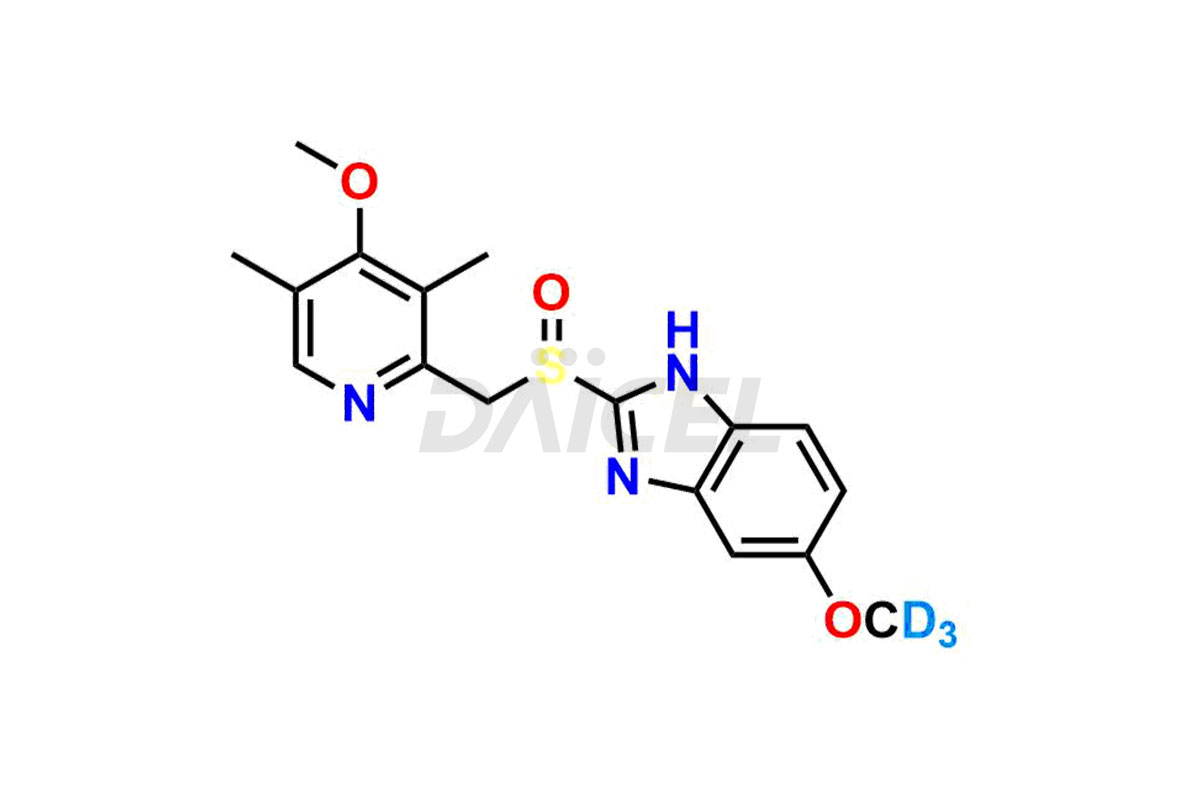
Omeprazole
General Information
Omeprazole Impurities and Omeprazole
Daicel Pharma provides Omeprazole impurities such as Omeprazole EP Impurity A, Omeprazole EP Impurity C, and Omeprazole EP impurity F&G mixture. These impurities are crucial in assessing Omeprazole quality, stability, and safety. Omeprazole impurities are specifically synthesized and distributed globally by Daicel Pharma.
Omeprazole [CAS: 73590-58-6] is a proton pump inhibitor (PPI) treating gastric acid-related disorders. Omeprazole functions as a Proton Pump Inhibitor and a Cytochrome P450 2C19 Inhibitor.
Omeprazole: Use and Commercial Availability
Omeprazole treats duodenal ulcers, benign gastric ulcers, gastroesophageal reflux disease (GERD), heartburn, other GERD symptoms, and erosive esophagitis.
Omeprazole is a proton pump inhibitor (PPI) prescribed for the following purposes: Treatment of current duodenal ulcers in adults; Helicobacter pylori eradication to lower the risk of duodenal ulcer recurrence in adults; Treatment of active benign gastric ulcer in adults, Treatment of symptomatic gastroesophageal reflux disease (GERD) in patients one year of age and older.
Omeprazole Structure and Mechanism of Action 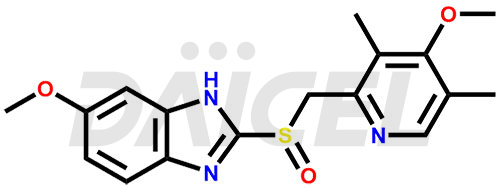
The chemical name of Omeprazole is 6-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole. Its chemical formula is C17H19N3O3S, and its molecular weight is approximately 345.4 g/mol.
Omeprazole suppresses gastric acid secretion by inhibiting the H+/K+ ATPase enzyme system.
Omeprazole Impurities and Synthesis
Numerous impurities can occur during the formation1 of Omeprazole. They can come from many sources, such as incomplete reactions, unwanted side reactions, or the starting materials or reagents utilized. Furthermore, impurities might occur during the storage or degradation of Omeprazole.
Daicel Pharma provides a Certificate of Analysis (CoA) of Omeprazole impurity standards such as Omeprazole EP Impurity A, Omeprazole EP Impurity C, and Omeprazole EP impurity F&G mixture. Daicel Pharma delivers a Certificate of Analysis (CoA) from a cGMP-certified analytical facility for Omeprazole impurity standards. Our CoA is from a cGMP-compliant analytical laboratory and includes characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we can give further characterization data, such as 13C-DEPT. Our experts supply extraordinarily pure Omeprazole Labelled Standard, the deuterated-labeled standard of Omeprazole for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) tests.
References
FAQ's
References
- Junggren, Ulf Krister; Sjostrand, Sven Erik, Substituted Pyridylsulfinylbenzimidazoles Having Gastric Acid Secretion Properties, Pharmaceutical Preparations Containing Same, And Intermediates For Their Preparation, Aktiebolag Hassle, Sweden, EP5129B1, April 29, 1981
- Lagerstroem, Per Olof; Persson, Bengt Arne, Determination of omeprazole and metabolites in plasma and urine by liquid chromatography, Journal of Chromatography, Biomedical Applications, Volume: 309, Issue: 2, Pages: 347-56, 1984
Frequently Asked Questions
Are there specific impurities of concern in Omeprazole?
Specific impurities in Omeprazole can vary depending on the manufacturing process and the drug product. Common Omeprazole impurities include related substances, degradation products, and residual solvents. Some impurities may have known toxicity or the potential to affect the drug's performance, and their levels are closely monitored and controlled.
Can impurities in Omeprazole be eliminated?
It is challenging to eliminate all impurities in any pharmaceutical product, including Omeprazole. However, manufacturers strive to minimize them through optimized synthesis processes, purification techniques, and stringent quality control. The aim is to keep impurity levels well below the acceptable limits defined by regulatory authorities to ensure the safety and efficacy of Omeprazole.
How are Omeprazole impurities formed?
Impurities in Omeprazole can occur through many methods during its production, storage, or use. Impurities can form during the synthetic process due to incomplete reactions, unwanted side reactions, or contaminants in the starting materials or reagents utilized.
What are the temperature conditions required to store Omeprazole impurities?
Omeprazole Impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.