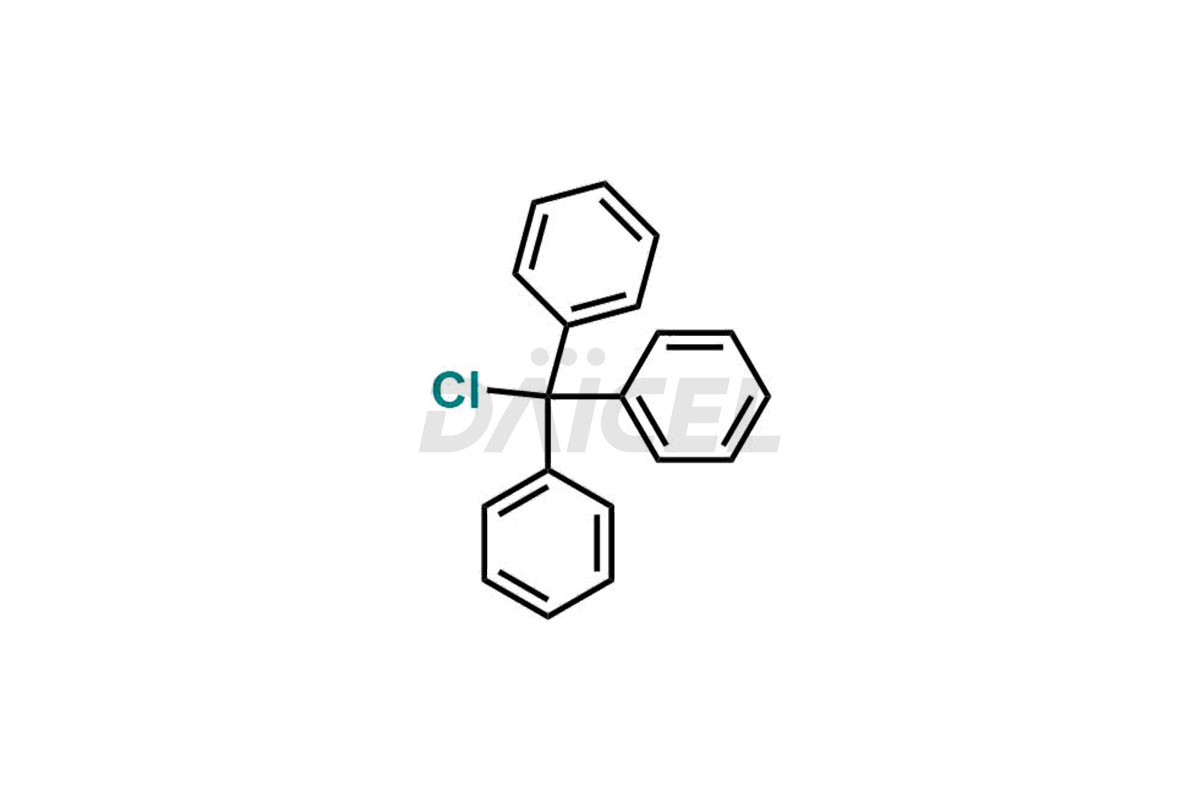
Olmesartan
General Information
Olmesartan Impurities and Olmesartan
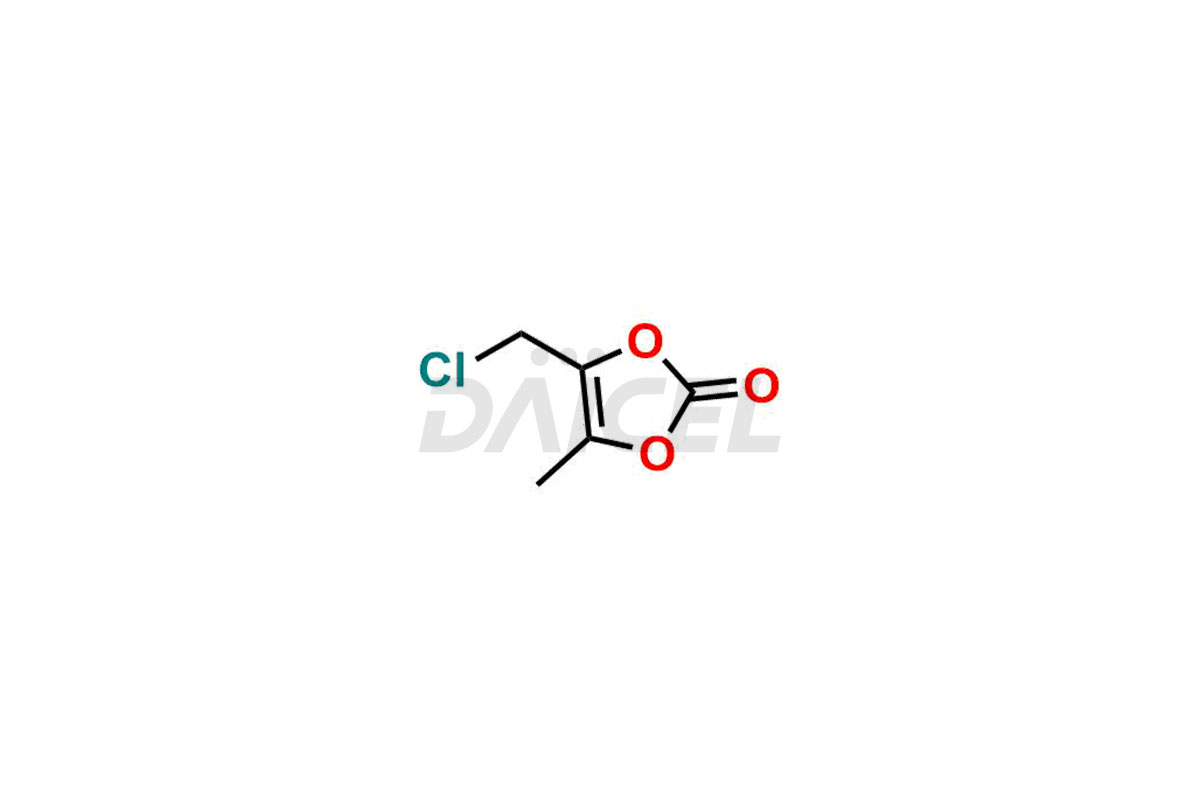
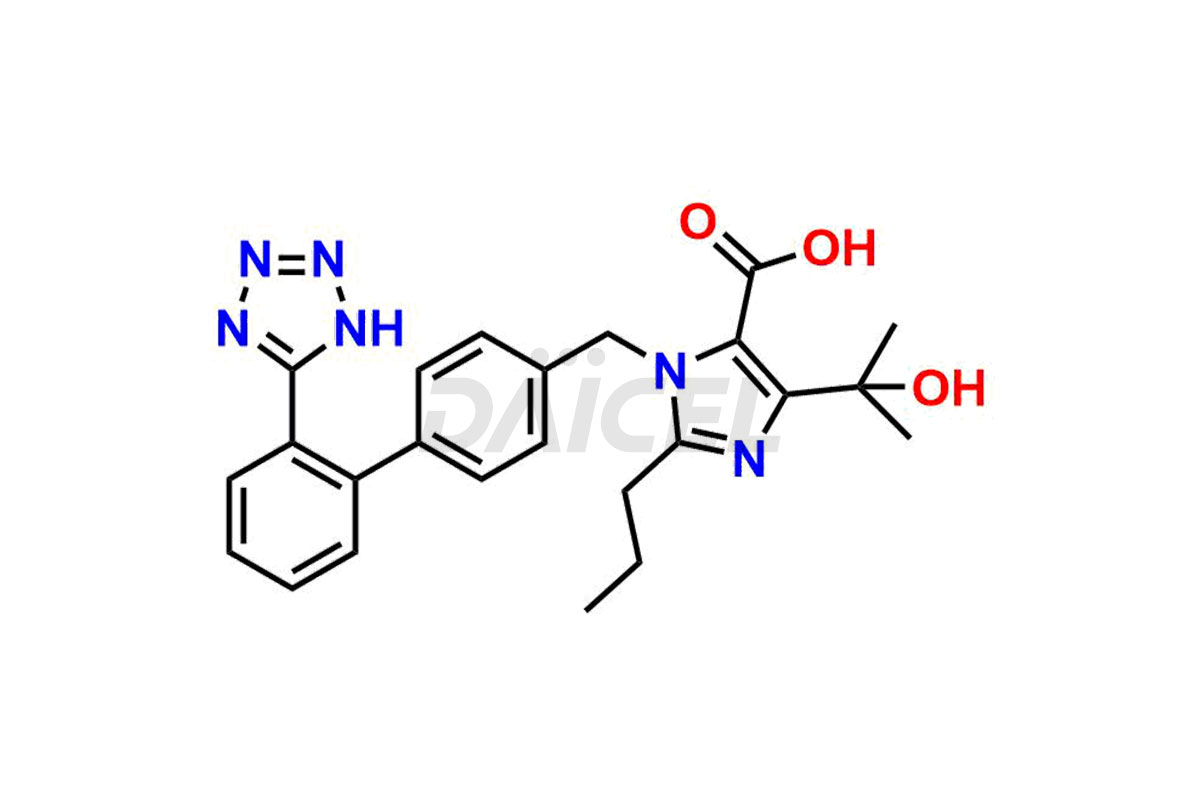
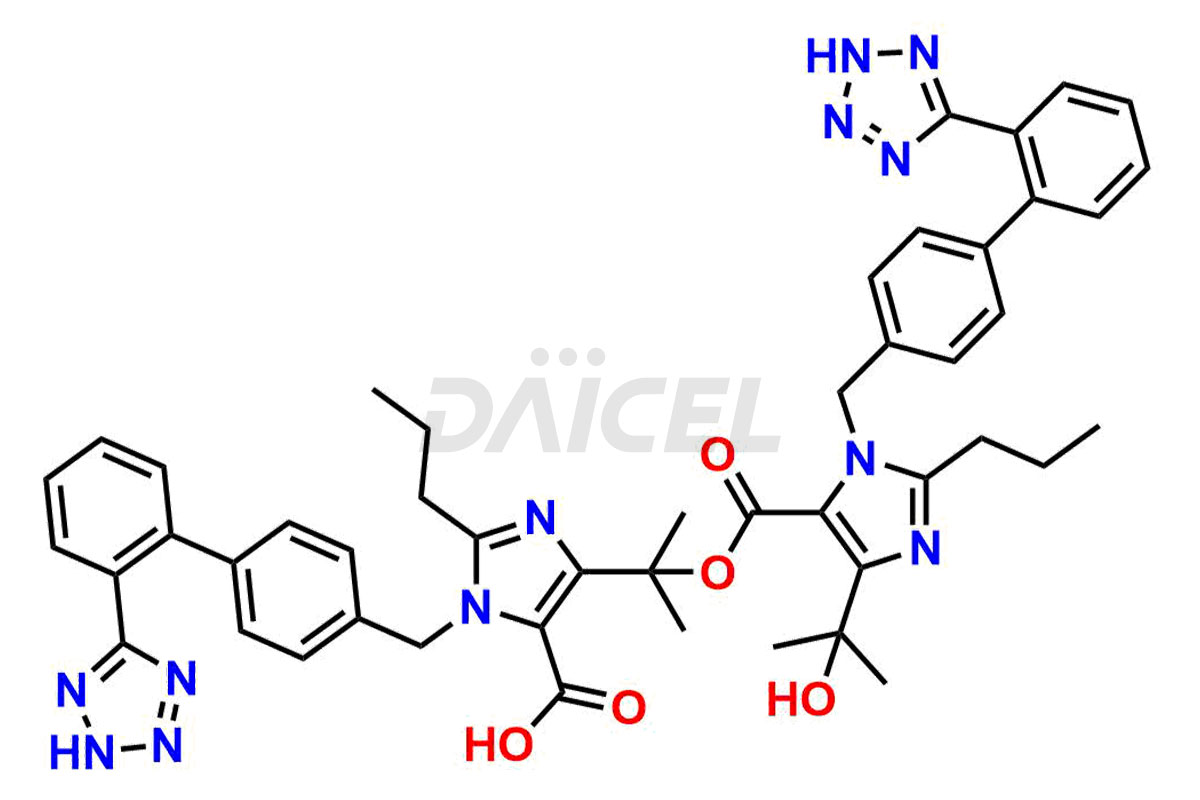
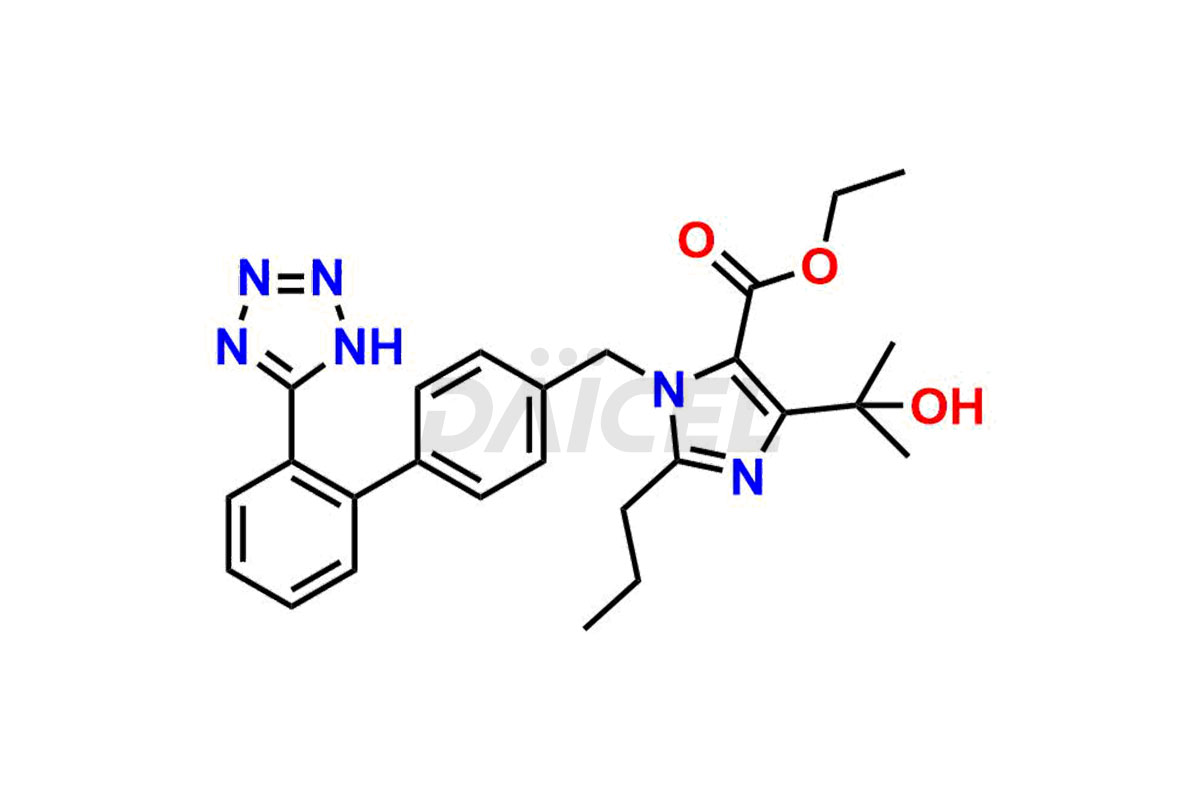
Daicel Pharma synthesizes high-quality Olmesartan impurities like 4-(chloromethyl)-5-methyl-2-oxo-1,3-dioxolene, Olmesartan Acid, Olmesartan Dimer Impurity, Olmesartan Ethyl Ester Impurity, Olmesartan: OMJ-2-Ethyl Impurity, and triphenylmethyl chloride, which are crucial in the analysis of the quality, stability, and biological safety of the active pharmaceutical ingredient Olmesartan. Moreover, Daicel Pharma offers custom synthesis of Olmesartan impurities and delivers them globally.
Olmesartan [CAS: 144689-24-7] is a selective angiotensin II receptor antagonist that belongs to the synthetic imidazole derivatives. It is commonly used to treat hypertension and has antihypertensive properties. Olmesartan, similar to other ARBs, can be used as a standalone treatment for hypertension when no comorbidities are present.
Olmesartan: Use and Commercial Availability
Olmesartan is a medicine used to treat hypertension alone or combined with other antihypertensive agents. It has also managed Type 2 Diabetes-associated nephropathy, heart failure, and post-myocardial infarction in patients who cannot tolerate ACE inhibitors. Azor, Benicar, Benicar Hct, and Tribenzor are some brand names of Olmesartan.
Olmesartan Structure and Mechanism of Action 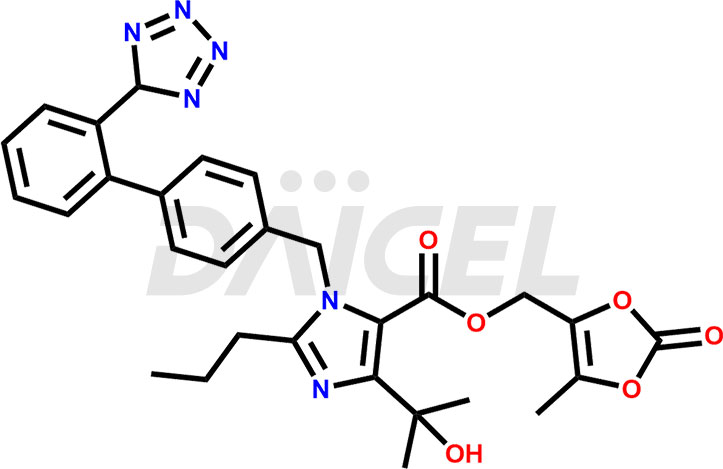
The chemical name of Olmesartan is 4-(1-Hydroxy-1-methylethyl)-2-propyl-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-carboxylic acid. Its chemical formula is C24H26N6O3, and its molecular weight is approximately 446.5 g/mol.
Olmesartan blocks the vasoconstrictor effects of angiotensin II by selectively preventing the binding of angiotensin II to the AT1 receptor in vascular smooth muscle.
Olmesartan Impurities and Synthesis
Olmesartan impurities form during the synthesis1, storage, or degradation. Common impurities found in Olmesartan include related substances, degradation products, and residual solvents. The impurities occur due to the reaction of Olmesartan with moisture, acids, bases, or other reactive species. The formation of impurities affects the drug’s stability, purity, and efficacy. Hence, strict control measures are necessary to ensure the quality of Olmesartan’s finished product.
Daicel offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Olmesartan impurity standards, 4-(chloromethyl)-5-methyl-2-oxo-1,3-dioxolene, Olmesartan Acid, Olmesartan Dimer Impurity, Olmesartan Ethyl Ester Impurity, Olmesartan: OMJ-2-Ethyl Impurity, and triphenylmethyl chloride. The CoA includes complete characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We also provide 13C-DEPT and CHN if requested. We give a complete characterization report on delivery. Daicel has the technology and expertise to prepare any unknown Olmesartan impurity or degradation product.
References
FAQ's
References
- Yanagisawa, Hiroaki; Shimoji, Yasuo; Fujimoto, Koichi; Kanazaki, Takuro; Anemiya, Yoshiya; Koike, Hiroyuki; Sada, Toshio, 1-Biphenylimidazole derivatives, their preparation and their therapeutic use, Sankyo Co., Ltd., Japan, EP503785B1, Feb 21, 1992
- Liu, Dongyang; Hu, Pei; Matsushima, Nobuko; Li, Xiaoming; Li, Li; Jiang, Ji, Quantitative determination of olmesartan in human plasma and urine by liquid chromatography coupled to tandem mass spectrometry, Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Volume: 856, Issue: 1-2, Pages: 190-197, 2007
Frequently Asked Questions
What are the sources of Olmesartan impurities?
Olmesartan impurities occur from many sources, such as the starting materials, manufacturing, and storage conditions.
Can the presence of impurities in Olmesartan be harmful to patients?
Olmesartan impurities can potentially pose a risk to patient health, as these impurities may have toxic or carcinogenic properties in the finished product.
Which solvent helps in the analysis of impurities in Olmesartan?
Methanol is a solvent that analyzes most of the Olmesartan impurities.
What are the temperature conditions required to store Olmesartan impurities?
Olmesartan impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.