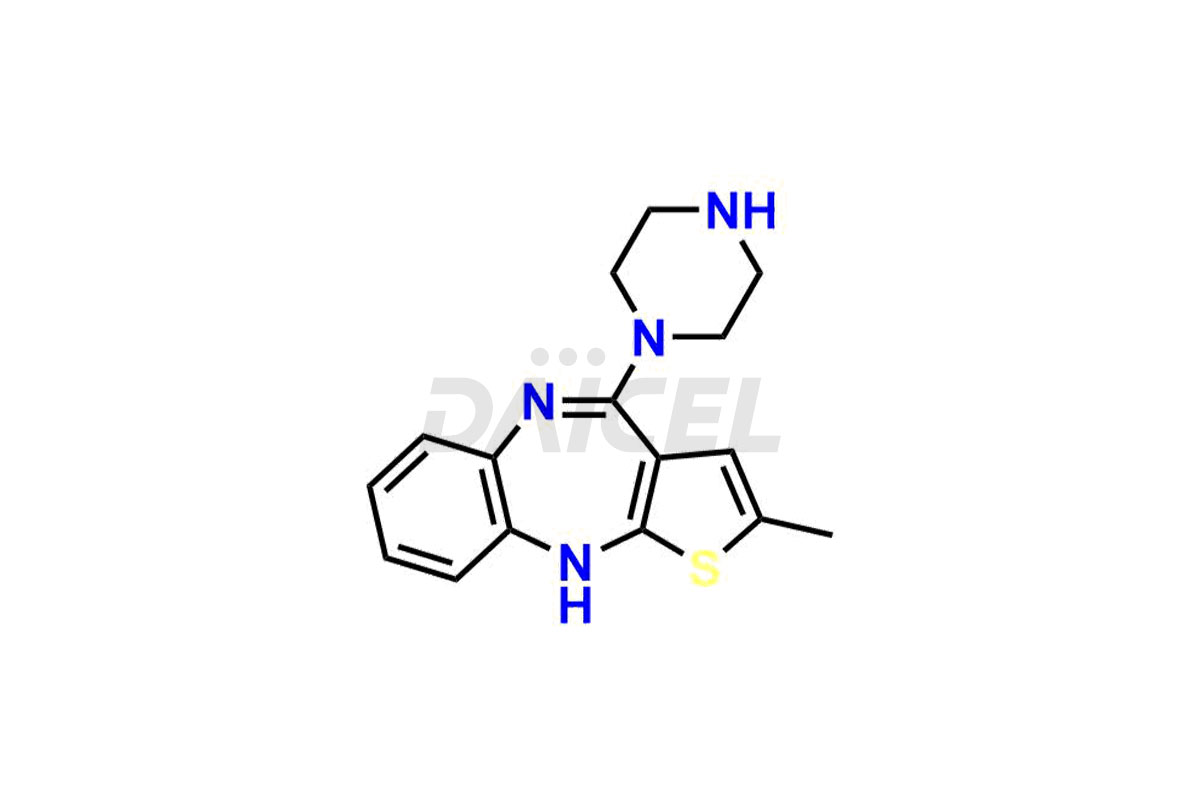
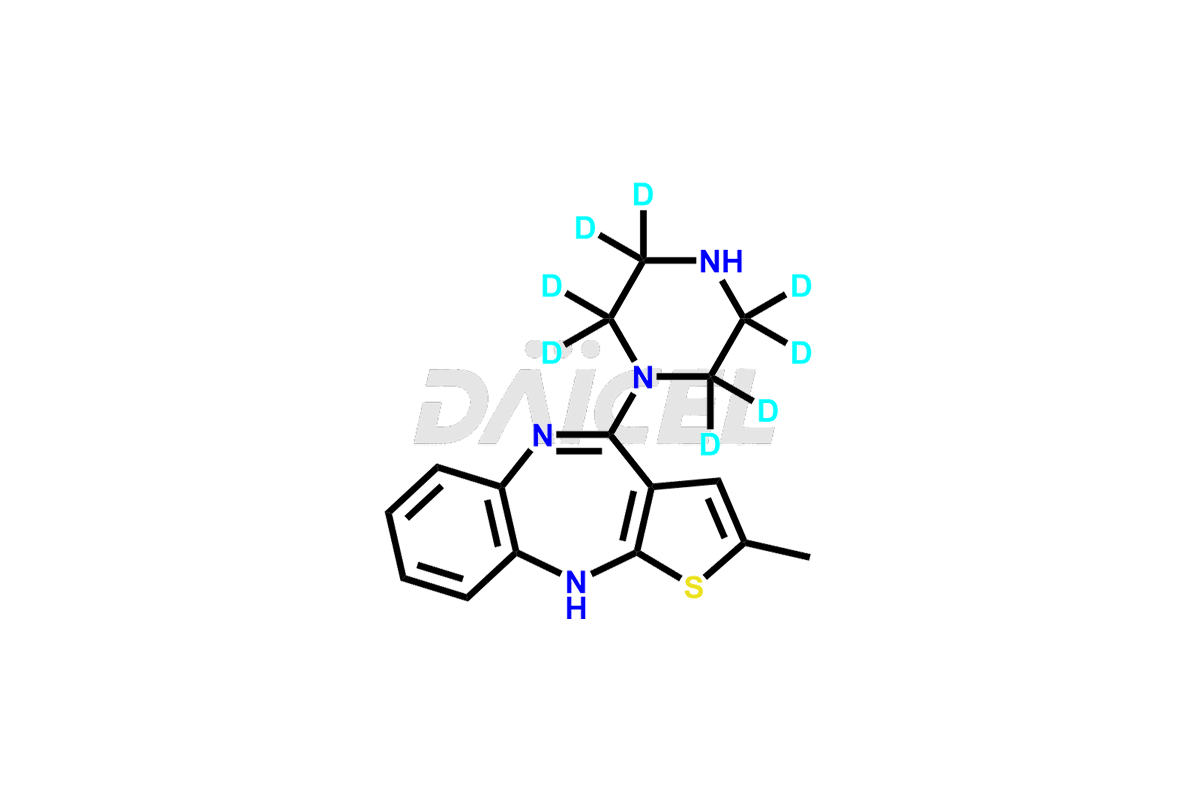
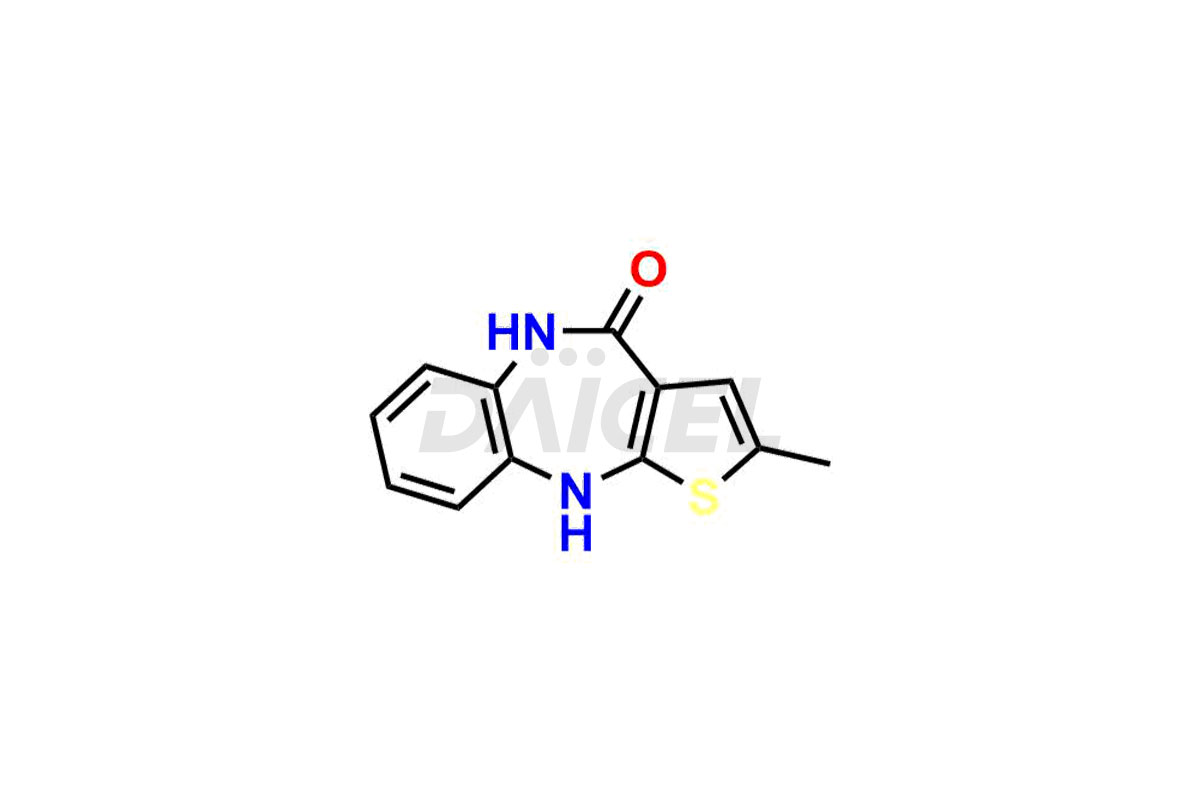
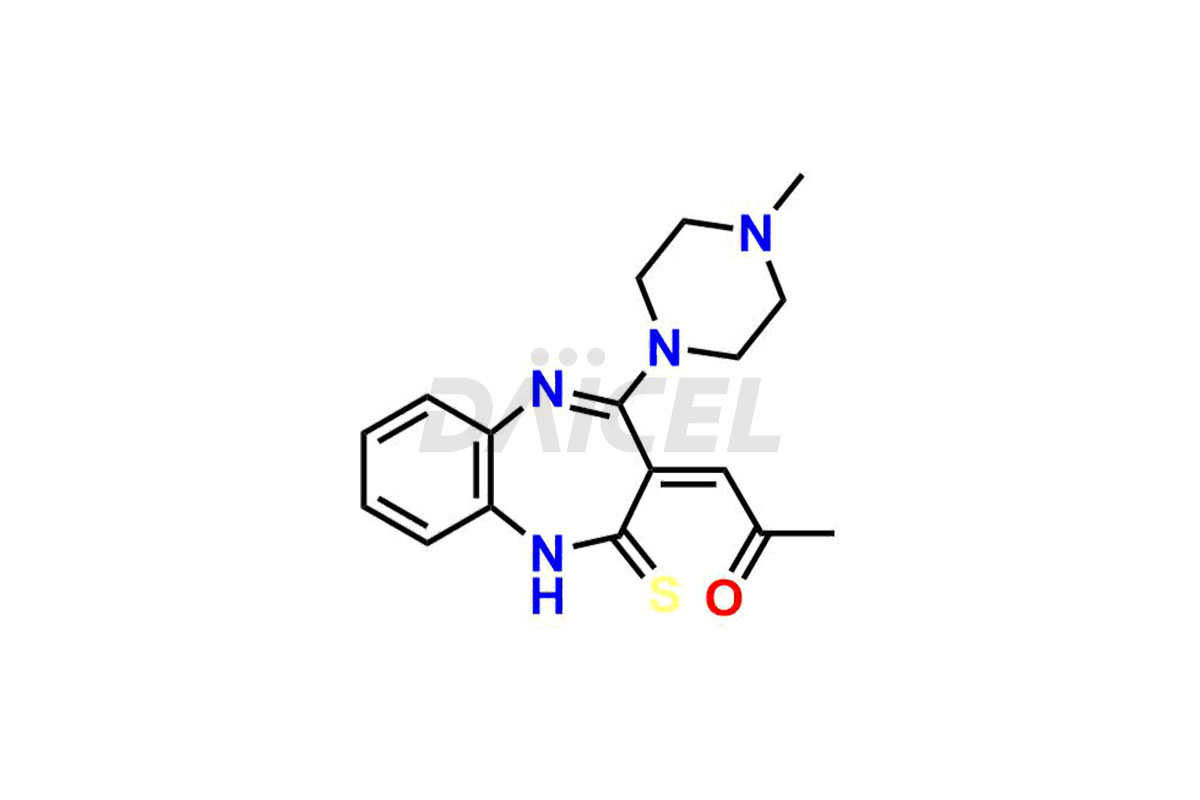
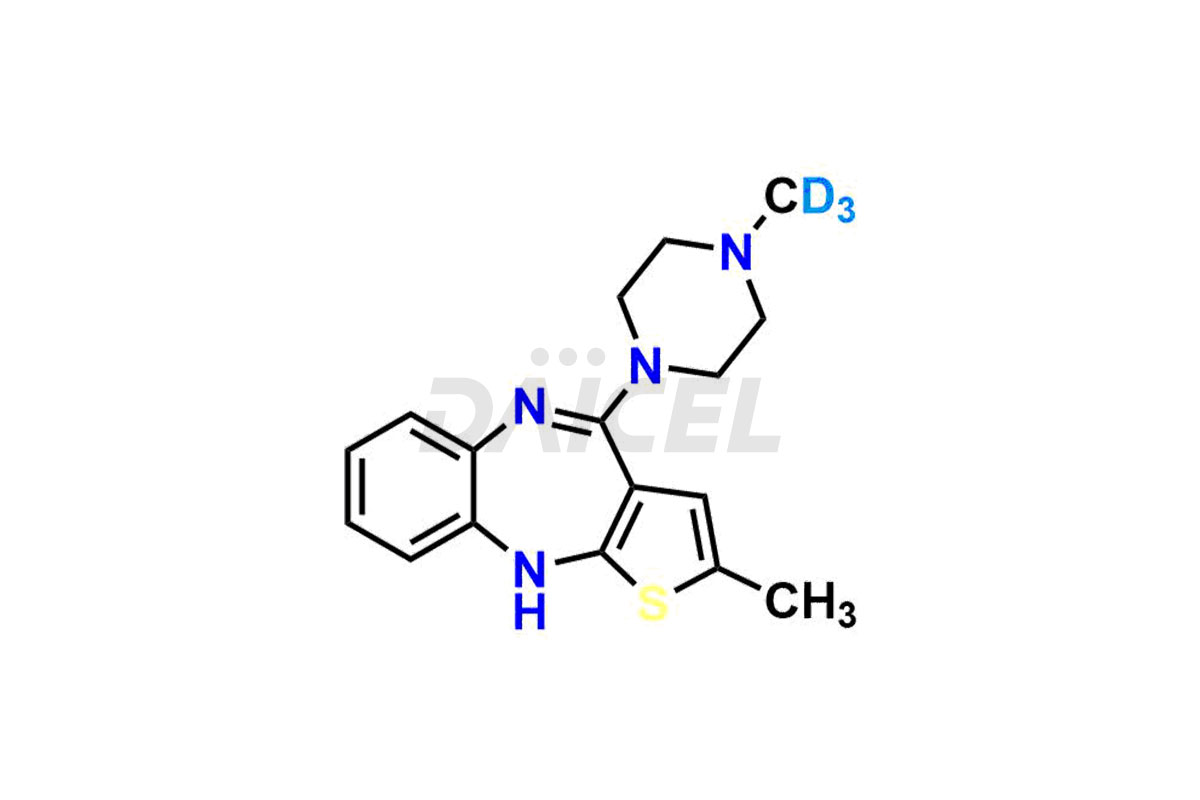
Olanzapine
General Information
Olanzapine Impurities and Olanzapine
Daicel Pharma offers Olanzapine impurities such as N-Demethyl Olanzapine, Olanzapine BP Impurity-3, Olanzapine degradation impurities 1 and 2, Olanzapine Related Compound A, Olanzapine Related Compound B, and Olanzapine Thiolactam Impurity. These impurities are critical for determining the quality, stability, and safety of Olanzapine, an active pharmaceutical component. Daicel Pharma custom synthesizes and distributes Olanzapine impurities globally.
Olanzapine [CAS: 132539-06-1] is a thienobenzodiazepine derivative. It functions as a histamine antagonist, muscarinic antagonist, serotonin antagonist, dopaminergic antagonist, antiemetic, second-generation antipsychotic, and serotonin uptake inhibitor. It contains benzodiazepines, N-methylpiperazines, and N-arylpiperazines.
Olanzapine: Use and Commercial Availability
Olanzapine’s use orally and intramuscularly treats persistent schizophrenia and other mental diseases, such as bipolar disorder, including mixed or manic episodes in patients. Olanzapine also has approval for the short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults when combined with lithium or valproate. Olanzapine treats moderate to severe manic episodes. Olanzapine prevents recurrence in patients with bipolar disorder whose manic episode has responded to Olanzapine therapy. Olanzapine is available as Zyprexa, Zyprexa Zydis, and Zyprexa Relprevv.
Olanzapine Structure and Mechanism of Action 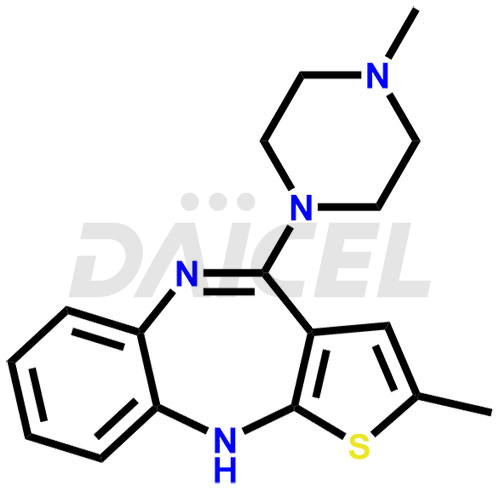
The chemical name of Olanzapine is 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine. Its chemical formula is C17H20N4S, and its molecular weight is approximately 312.4 g/mol.
The mechanism of action of Olanzapine is unknown concerning other drugs.
Olanzapine Impurities and Synthesis
Olanzapine impurities are unwanted substances that can be present in the drug formulation of Olanzapine. They can arise during synthesis1, storage, or degradation processes. Controlling impurities is essential for maintaining the quality and effectiveness of Olanzapine.
Daicel provides a Certificate of Analysis (CoA) of Olanzapine impurities such as N-Demethyl Olanzapine, Olanzapine BP Impurity-3, Olanzapine degradation impurities 1 and 2, Olanzapine Related Compound A, Olanzapine Related Compound B, and Olanzapine Thiolactam Impurity. Our cGMP-compliant analytical laboratory offers the CoA, which contains extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, such as 13C-DEPT, on request. Fortunately, Daicel Pharma has a team of specialists to offer Olanzapine unknown impurities. Our professionals provide exceptionally pure Olanzapine-D3, deuterium-labeled standards of Olanzapine that are essential for bioanalytical research and Bioavailability/Bioequivalence (BA/BE) studies.
References
FAQ's
References
- Chakrabarti, Jiban Kumar; Hotten, Terrence Michael; Tupper, David Edward, Pharmaceutical compounds, Lilly Industries Ltd., United Kingdom, EP454436B1, September 13, 1995
- Catlow, John T.; Barton, Richard D.; Clemens, Matt; Gillespie, Todd A.; Goodwin, Michael; Swanson, Steven P., Analysis of olanzapine in human plasma utilizing reversed-phase high-performance liquid chromatography with electrochemical detection, Journal of Chromatography B: Biomedical Sciences and Applications, Volume: 668, Issue: 1, Pages: 85-90,1995
Frequently Asked Questions
How are Olanzapine Impurities controlled?
During manufacturing, strict quality control procedures help manage Olanzapine impurities. Impurity levels are subject to regulatory bodies' limitations.
How are Olanzapine impurities formed?
During the synthetic process of Olanzapine, unintended chemical reactions or side reactions can occur, leading to the formation of impurities. These impurities can result from incomplete reactions, unexpected intermediates, or variations in reaction conditions.
Can Olanzapine Impurities be removed?
During synthesis, Olanzapine impurities are controlled by utilizing established analytical techniques, monitoring reaction conditions, and adopting quality control measures.
What are the temperature conditions required to store Olanzapine Impurities?
Olanzapine impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.