Obeticholic acid
General Information
Obeticholic Acid Impurities and Obeticholic Acid
Daicel Pharma offers Obeticholic Acid impurities such as Obeticholic acid Methyl Ester, Obeticholic Acid, OCA 3 Glucuronide, Stage-III Obeticholic acid, etc. These impurities are essential for evaluating the quality, stability, and safety of Obeticholic Acid, an active pharmaceutical ingredient. Moreover, Daicel Pharma provides custom synthesis of Obeticholic Acid impurities and distributes them internationally to fulfill the client’s needs.
Obeticholic Acid [CAS: 459789-99-2] is a semisynthetic bile acid that treats primary biliary cholangitis. It is a farnesoid X receptor agonist and a hepatoprotective drug. It is structurally similar to chenodeoxycholic acid.
Obeticholic Acid: Use and Commercial Availability
Obeticholic acid treats primary biliary cholangitis (PBC) with ursodeoxycholic acid (UDCA) for adults who have not responded adequately to UDCA alone. It is used for adults with PBC unable to tolerate UDCA. Obeticholic acid is available as Ocaliva.
Obeticholic Acid Structure and Mechanism of Action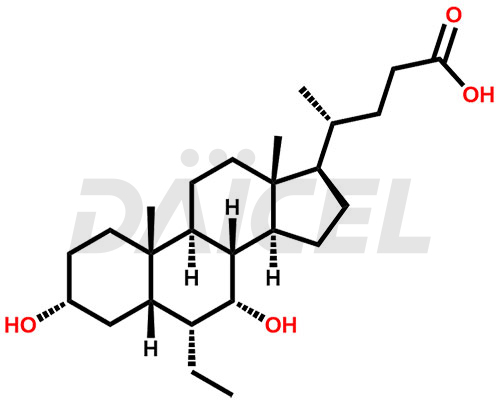
The chemical name of Obeticholic acid is (3α,5β,6α,7α)-6-Ethyl-3,7-dihydroxycholan-24-oic acid. Its chemical formula is C26H44O4, and its molecular weight is approximately 420.6 g/mol.
Obeticholic acid binds to the farnesoid X receptor (FXR), which regulates bile acid. It decreases hepatic exposure to bile acids.
Obeticholic Acid Impurities and Synthesis
Obeticholic Acid (OCA) impurities refer to undesired substances that may be present in the drug formulation of Obeticholic Acid. These impurities can arise during the synthetic process1 or storage and include related compounds, degradation products, and residual solvents. Controlling and monitoring them is essential to ensure the quality, safety, and efficacy of Obeticholic Acid.
Daicel provides a Certificate of Analysis (CoA) of Obeticholic Acid impurity standards like Obeticholic acid Methyl Ester, Obeticholic Acid, OCA 3 Glucoronide, Stage-III obeticholic acid. Our cGMP-compliant analytical laboratory provides the CoA, which contains extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. We give additional characterization data, such as 13C-DEPT, on request. Daicel Pharma has the expertise and capabilities to offer unknown impurities or degradation products of Obeticholic Acid.
References
FAQ's
References
- Pellicciari, Roberto; Fiorucci, Stefano; Camaioni, Emidio; Clerici, Carlo; Costantino, Gabriele; Maloney, Patrick R.; Morelli, Antonio; Parks, Derek J.; Willson, Timothy M., 6α-Ethyl-Chenodeoxycholic Acid (6-ECDCA), a Potent and Selective FXR Agonist Endowed with Anticholestatic Activity, Journal of Medicinal Chemistry, Volume: 45, Issue: 17, Pages: 3569-3572, 2002
- Dousa, Michal; Slavikova, Marketa; Kubelka, Tomas; Cerny, Josef; Gibala, Petr; Zezula, Josef, HPLC/UV/MS method application for the separation of obeticholic acid and its related compounds in development process and quality control, Journal of Pharmaceutical and Biomedical Analysis, Volume: 149, Pages: 214-224, 2018
Frequently Asked Questions
Are generic versions of Obeticholic acid tested for Impurities?
Yes, generic Obeticholic acid is thoroughly tested for impurities to assure quality, safety, and efficacy. Comparative studies and thorough quality control help guarantee that generic versions are equal in purity and drug efficacy.
Can Obeticholic Acid Impurities affect its effectiveness?
Obeticholic Acid impurities may reduce its efficacy by affecting its pharmacokinetics, pharmacodynamics, or stability. Impurity control and monitoring ensure the medicine retains its therapeutic efficacy and achieves the targeted therapeutic effects.
How are Obeticholic acid Impurities controlled?
Impurities in Obeticholic acid are managed during production using strict quality control procedures. Regulatory agencies impose specific limitations on impurity levels.
What are the temperature conditions required to store Obeticholic Acid Impurities?
Obeticholic Acid impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.

![Obeticholic acid-[PEG]-n-Ester](https://dev.daicelpharmastandards.com/wp-content/uploads/2022/06/DCTI-C-1864-900x600.jpg)
