Nitroso Compounds
General Information
Nitroso Compounds Impurities and Nitroso Compounds
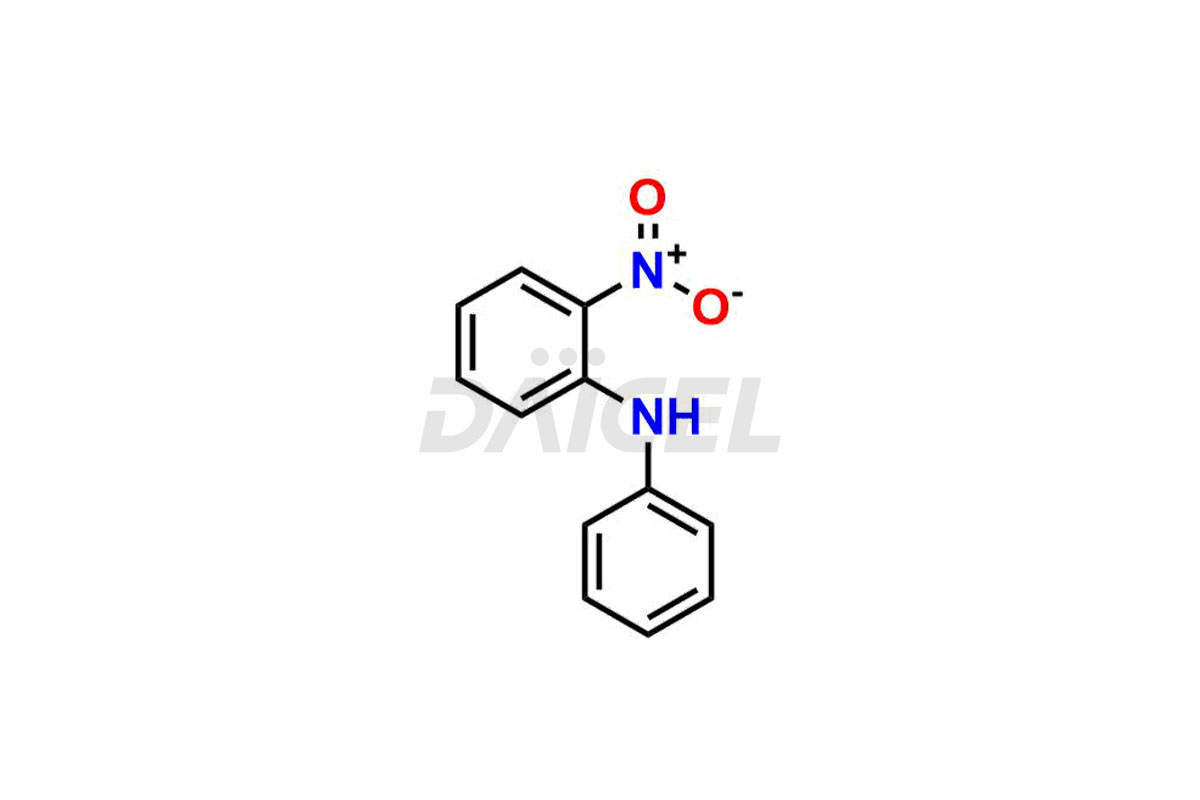
Daicel Pharma offers Nitroso Compounds impurities, including 1-nitroso-1,2,3,6-tetrahydropyridine, 2-Nitro-diphenylamine (NDPA), N-methyl-N-nitrosophenethylamine, N-Nitroso Dabigatran etexilate, N-Nitroso Mirabegron, N-Nitroso N-Desbenzyl Nicardipine Impurity, Palbociclib Nitroso Impurity, N-Nitroso Rasagiline, N-(2-(ethyl(nitroso)amino)ethyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-Nitroso Tigecycline, and more. These impurities are essential for evaluating the quality, stability, and safety of Nitroso Compounds. Furthermore, Daicel Pharma offers custom synthesis of Nitroso Compounds impurities and distributes them internationally to fulfill the clientele’s needs.
Nitroso Compounds have varied applications, including intermediates in organic synthesis, catalysts, and pharmaceutical agents.
Nitroso Compounds: Use and Commercial Availability
Nitroso compounds can be useful as intermediates in synthesizing pharmaceuticals, agrochemicals, dyes, and other chemical compounds. They can also act as catalysts in chemical reactions. Nitroso Compounds are available under the Monograph System governed by OTC Monographs and Administrative Orders.
Nitroso Compounds Impurities and Synthesis
Nitroso Compounds impurities refer to unwanted substances that arise during the synthetic process or storage and may include unreacted starting materials, by-products, or degradation products. The control and monitoring of impurities assure the quality, safety, and efficacy of Nitroso Compounds. The synthesis of Nitroso Compounds involves various methods depending on the specific compound desired.
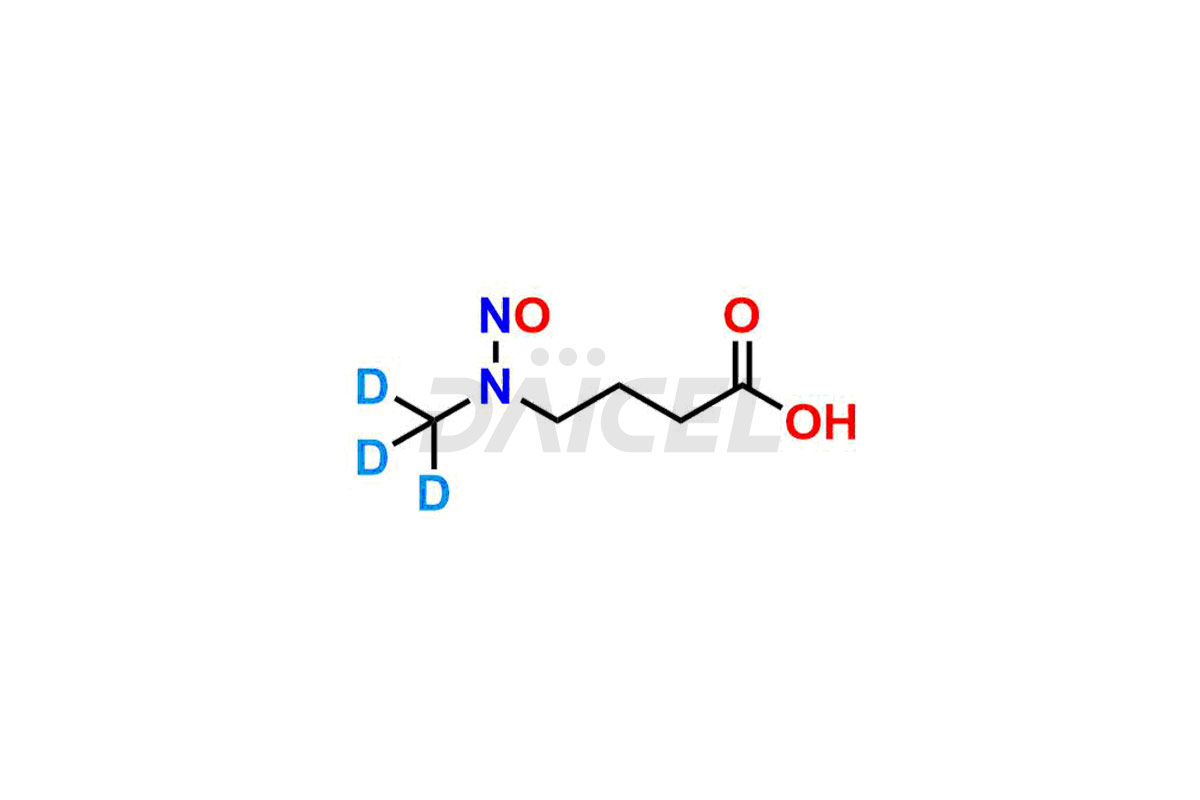
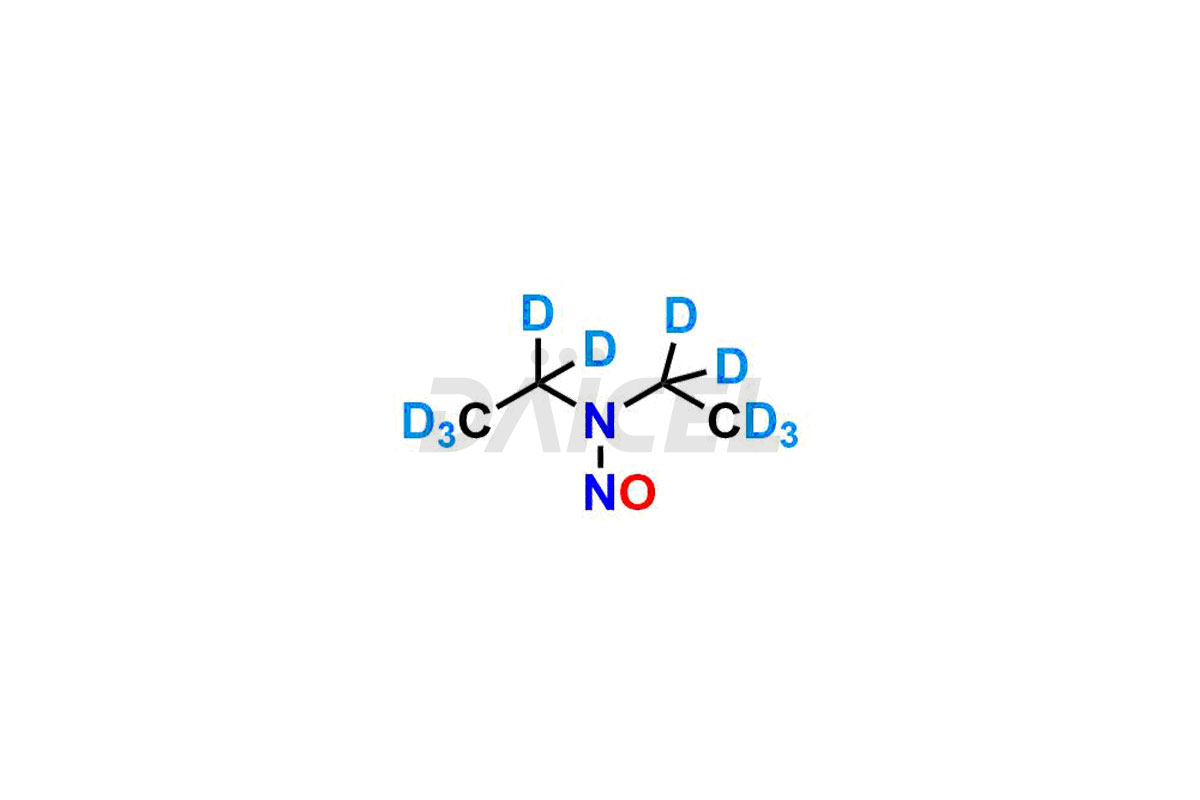
Daicel provides a Certificate of Analysis (CoA) for Nitroso Compound impurity standards, including 1-nitroso-1,2,3,6-tetrahydropyridine, 2-Nitro-diphenylamine(NDPA), and N-methyl-N-nitrosophenethylamine that can be present in Nitroso Compounds. Also, Nitroso impurity standards like N-Nitroso Dabigatran etexilate, N-Nitroso Mirabegron, N-Nitroso N-Desbenzyl Nicardipine Impurity, Palbociclib Nitroso Impurity, N-Nitroso Rasagiline, N-(2-(ethyl(nitroso)amino)ethyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-Nitroso Tigecycline, and more. Our cGMP-compliant analytical laboratory offers the CoA, which contains extensive characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity1. We give additional characterization data, such as 13C-DEPT, on request. Daicel Pharma can synthesize unknown Nitroso Compound impurities or degradation products and deliver labeled compounds to test the efficacy of generic Nitroso Compounds. Each shipment includes a detailed characterization report. Daicel Pharma offers N-Nitroso-N-methyl-4-aminobutyric Acid-d3, Sitagliptin Nitroso Impurity-D4, Nitroso Nebivolol D4, Trimetazidine nitroso D9 impurity, etc., deuterium-labeled Nitroso compound standards for bioanalytical research and BA/BE testing.
References
FAQ's
Frequently Asked Questions
Why are Nitroso Compounds Impurities a concern?
Nitroso Compounds impurities can affect the drug's quality, safety, and efficacy. They may alter the chemical properties, stability, or toxicity leading to adverse effects or reduced effectiveness.
How are Nitroso Compounds Impurities controlled?
Strict quality control measures help during the manufacturing process of Nitroso compounds. Regulatory authorities set specific limits for impurity levels of compounds.
Can Nitroso Compounds Impurities impact generic versions?
Generic manufacturers must demonstrate that their products meet the same impurity standards as the branded version. Comparative studies and rigorous quality control ensure that generic versions are equivalent in drug purity, safety, and efficacy.
What are the temperature conditions required to store Nitroso Compounds Impurities?
Nitroso compound impurities are kept at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.