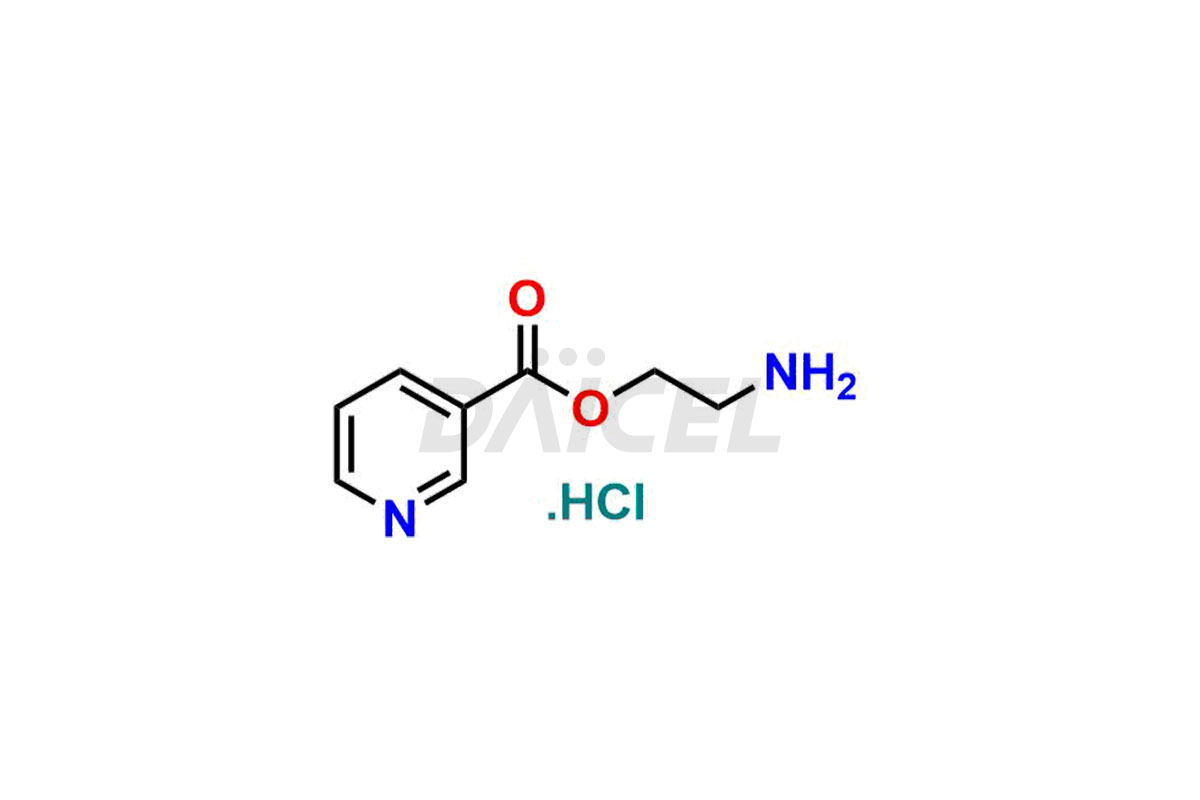
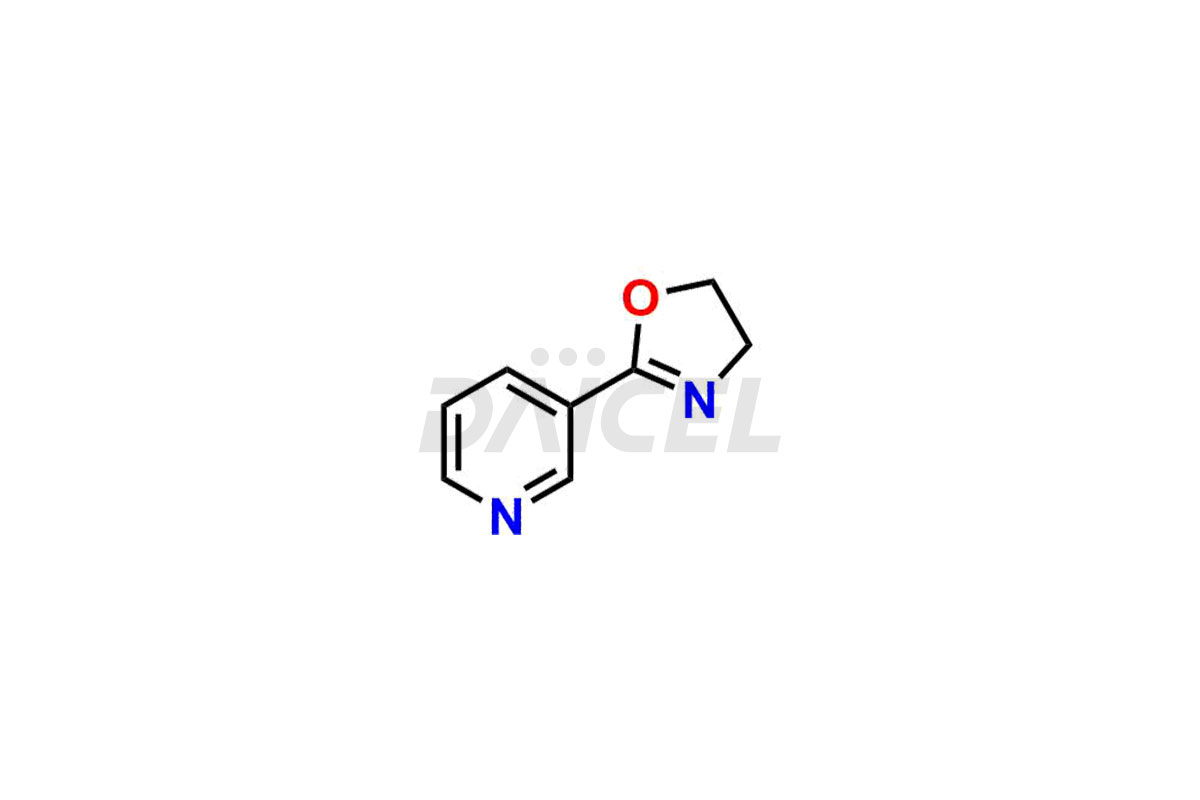
Nicorandil
General Information
Nicorandil Impurities and Nicorandil
Daicel Pharma offers Nicorandil impurity standards such as 2-Aminoethyl nicotinate HCl and Oxazoalyl pyridine, which play a vital role in assessing the purity, quality, and safety of Nicorandil. Daicel Pharma provides a custom synthesis of Nicorandil impurities to meet specific client needs, offering global delivery options for convenience.
Nicorandil [CAS: 65141-46-0] is a pyrimidine carboxamide derivative. It exhibits properties similar to nitrates and activates ATP-sensitive potassium channels, thus preventing and treating angina pectoris. Nicorandil functions as both a vasodilator and a potassium channel opener.
Nicorandil: Use and Commercial Availability
Nicorandil acts as a potassium channel opener and releases nitric oxide (NO). This dual action allows it to dilate both arterial and venous blood vessels. It treats or prevents cardiac arrhythmias. It is available under the tradename of Ikorel.
Nicorandil Structure and Mechanism of Action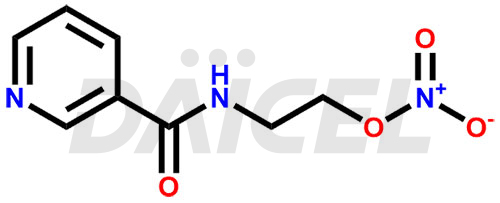
The chemical name of Nicorandil is N-[2-(Nitrooxy)ethyl]-3-pyridinecarboxamide. Its chemical formula is C8H9N3O4, and its molecular weight is approximately 211.18 g/mol.
Nicorandil activates and opens ATP-sensitive potassium channels composed of Kir6.x-type and sulfonylurea receptor subunits.
Nicorandil Impurities and Synthesis
Nicorandil impurities can arise during the manufacturing process1. They are of concern because they can impact the drug’s quality, effectiveness, and safety. Impurities can affect the drug’s stability and pharmacokinetics and cause harm. Extensive analysis and control of Nicorandil impurities are required to satisfy regulatory criteria and maintain patient safety. It comprises comprehensive impurity characterization and methods to reduce their formation during manufacture and storage.
Daicel Pharma offers a Certificate of Analysis (CoA) from a cGMP-compliant analytical facility for Nicorandil impurity standards which are 2-Aminoethyl nicotinate HCl and Oxazoalyl pyridine. Daicel Pharma offers a cGMP-certified analytical laboratory that provides the CoA, which includes thorough characterization data including 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. On request, we give more characterization details, such as those for 13C-DEPT. At Daicel Pharma, our team of experts is technically capable of synthesizing Nicorandil impurities and labeled compounds to evaluate generic Nicorandil.
References
FAQ's
References
- Nagano, Hiroyuki; Mori, Takashi; Takaku, Sakae; Matsunaga, Isao; Kujirai, Tatsuo; Ogasawara, Toshichika; Sugano, Shigeru; Shindo, Minoru, Nicotinamide Derivatives Processes For Producing The Same And Pharmaceutical Compositions Containing The Same, Chugai Pharmaceutical Co., Ltd., Japan, GB1562962A, March 19, 1980
- Tanikawa, Masahiko; Uzu, Megumi; Ohsawa, Yasutsugu; Fukushima, Masafumi, Sensitive method for determination of nicorandil in human plasma by reversed-phase high-performance liquid chromatography with ultraviolet detection, Journal of Chromatography, Biomedical Applications Volume: 617, Issue: 1, Pages: 163-7, 1993
Frequently Asked Questions
What is the origin of Nicorandil impurities?
The impurities in Nicorandil can originate from various sources, including the synthesis process, degradation of the drug, and interactions with other components or packaging materials.
How do Nicorandil impurities impact the drug's color, odor, or taste?
The presence of impurities in Nicorandil formulations can potentially affect their color, odor, or taste.
How do Nicorandil impurities relate to reference standards or compendial requirements?
Nicorandil impurities are compared to reference standards or compendial requirements to ensure compliance with established quality specifications and to assess the drug purity and quality.
What are the temperature conditions required to store Nicorandil impurities?
Nicorandil impurities are stored at a regulated room temperature of 2-8°C or as specified on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.