Neratinib
General Information
Neratinib Impurities and Neratinib
Daicel Pharma is a reliable source for synthesizing high-quality Neratinib impurity standards like
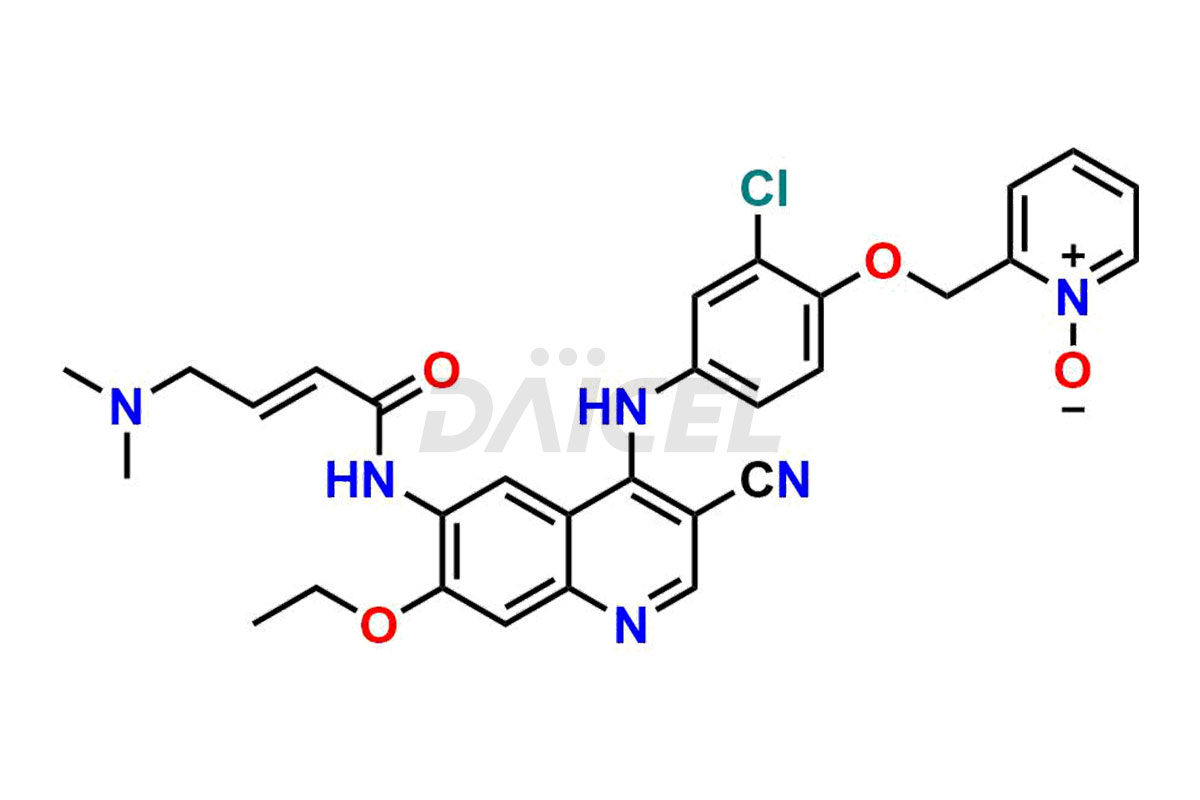
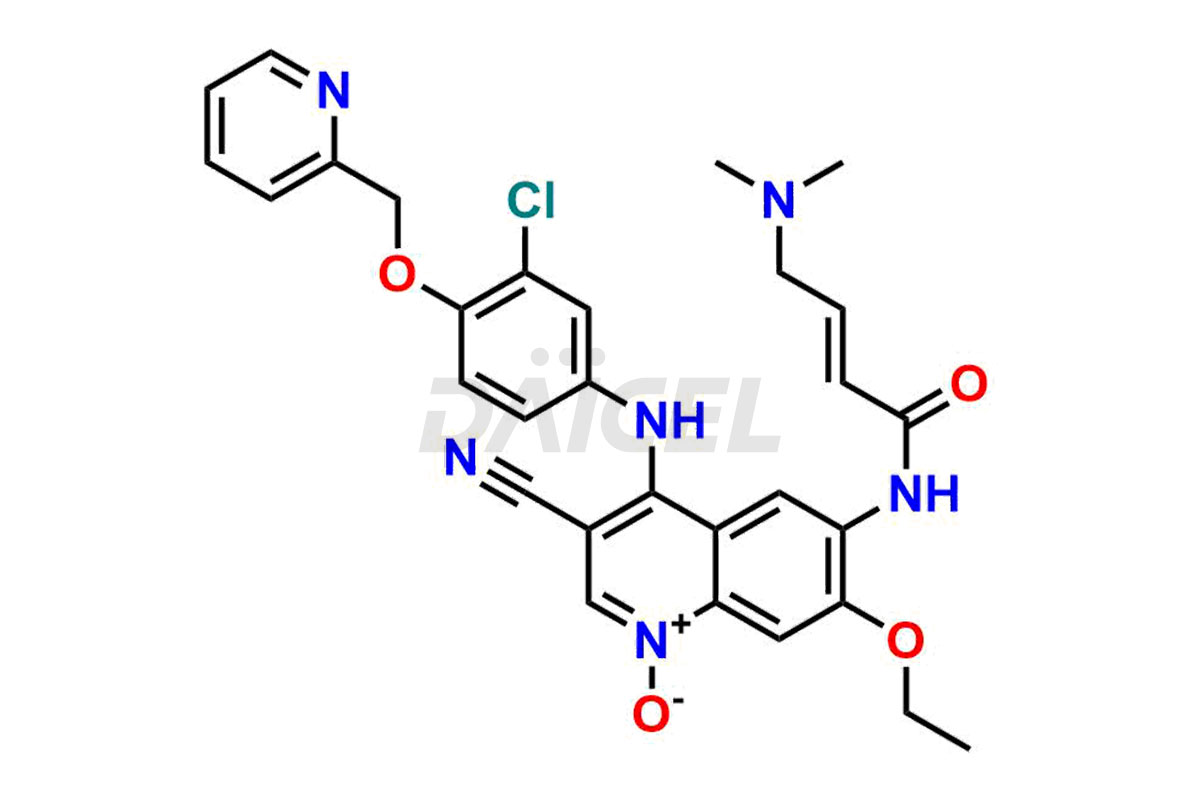
Neratinib pyridine N-oxide and Neratinib quinoline N-oxide. These impurities are essential in accurately analyzing the Neratinib quality, stability, and biological safety. Additionally, Daicel Pharma offers a customized synthesis of Neratinib impurities for global delivery to meet the specific needs of our customers.
Neratinib [CAS: 698387-09-6] is a protein kinase inhibitor. It treats adult patients with early-stage HER2-overexpressed or amplified breast cancer.
Neratinib: Use and Commercial Availability
Neratinib treats HER2-positive breast cancer. It also treats metastatic breast cancer. Neratinib can help slow down the progression of the disease and potentially improve overall survival rates in patients with HER2-positive metastatic breast cancer.
Neratinib is available under the brand name Nerlynx, which contains the active ingredient Neratinib.
Neratinib Structure and Mechanism of Action
The chemical name of Neratinib is (2E)-N-[4-[[3-Chloro-4-(2-pyridinylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-butenamide. Its chemical formula is C30H29ClN6O3, and its molecular weight is approximately 557.0 g/mol.
Neratinib inhibits Epidermal Growth Factor Receptor (EGFR), Human Epidermal Growth Factor Receptor 2 (HER2), and HER4 activity.
Neratinib Impurities and Synthesis
During Neratinib synthesis, impurities formation is possible, compromising its effectiveness. These impurities can arise from various sources, including the raw materials, intermediates, and chemicals utilized to synthesize Neratinib. Close management and monitoring of these impurities is essential to ensure optimal efficacy and safety of the drug.
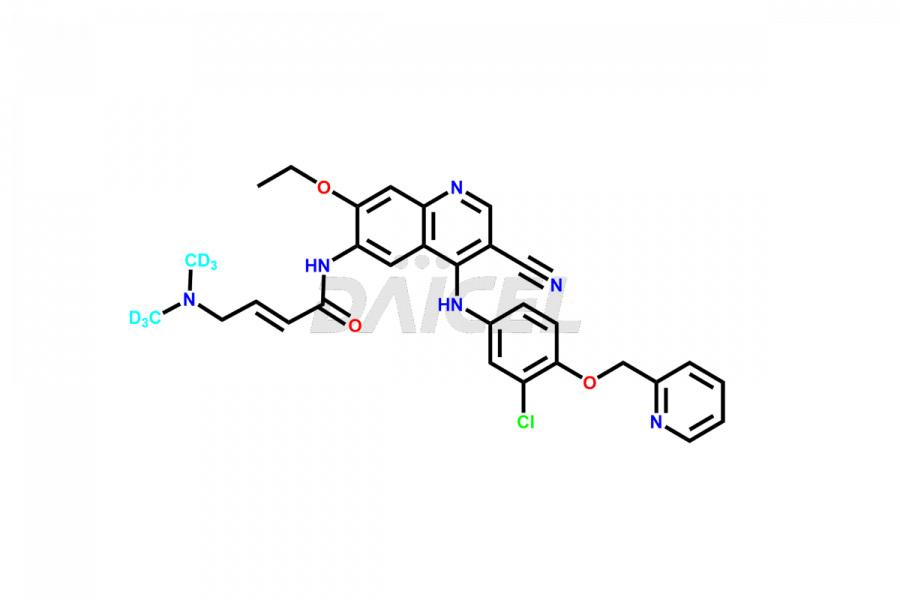
Daicel Pharma offers a wholesome and integrated Certificate of Analysis (CoA) for Neratinib impurity standards, encompassing Neratinib pyridine N-oxide and Neratinib quinoline N-oxide. The Certificate of Analysis (CoA) offers extensive detailed characterization data, including 1H NMR, 13C NMR, IR, MASS, and HPLC purity, ensuring comprehensive information about the product. Furthermore, Daicel Pharma possesses advanced technological capabilities and expertise to synthesize Neratinib’s unknown impurities or degradation products. We also provide Neratinib-D6, a deuterium-labeled compound of Neratinib.
References
FAQ's
References
- Tsou, Hwei-Ru; Overbeek-Klumpers, Elsebe G.; Hallett, William A.; Reich, Marvin F.; Floyd, M. Brawner; Johnson, Bernard D.; Michalak, Ronald S.; Nilakantan, Ramaswamy; Discafani, Carolyn; Golas, Jonathan; et al, Optimization of 6,7-Disubstituted-4-(arylamino)quinoline-3-carbonitriles as Orally Active, Irreversible Inhibitors of Human Epidermal Growth Factor Receptor-2 Kinase Activity, Journal of Medicinal Chemistry, Volume: 48, Issue: 4, Pages: 1107-1131, 2005
- Wania, Tanveer A.; Zargar, Seema; Ahmad, Ajaz, Ultra performance liquid chromatography tandem mass spectrometric method development and validation for determination of neratinib in human plasma, South African Journal of Chemistry, Volume: 68, Pages: 93-98, 2015
Frequently Asked Questions
Why is it essential to control impurities in Neratinib?
Controlling impurities in Neratinib is crucial to ensure their quality, safety, and efficacy.
What is the acceptable limit for Neratinib impurities?
Regulatory agencies like the US FDA, EMA, and ICH establish acceptable limits for impurities in Neratinib. Their limit may vary depending on the impurity level, ensuring that Neratinib meets stringent standards.
How are Neratinib impurities controlled during the manufacturing process of the drug?
Impurities in Neratinib are controlled during the synthetic process by implementing good manufacturing practices (GMP) and using appropriate analytical methods for quantification.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.