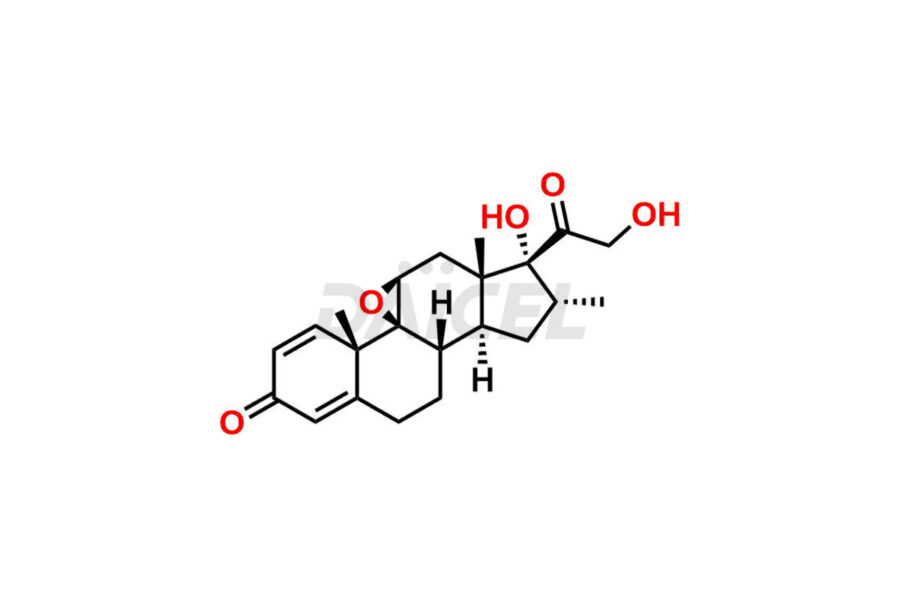
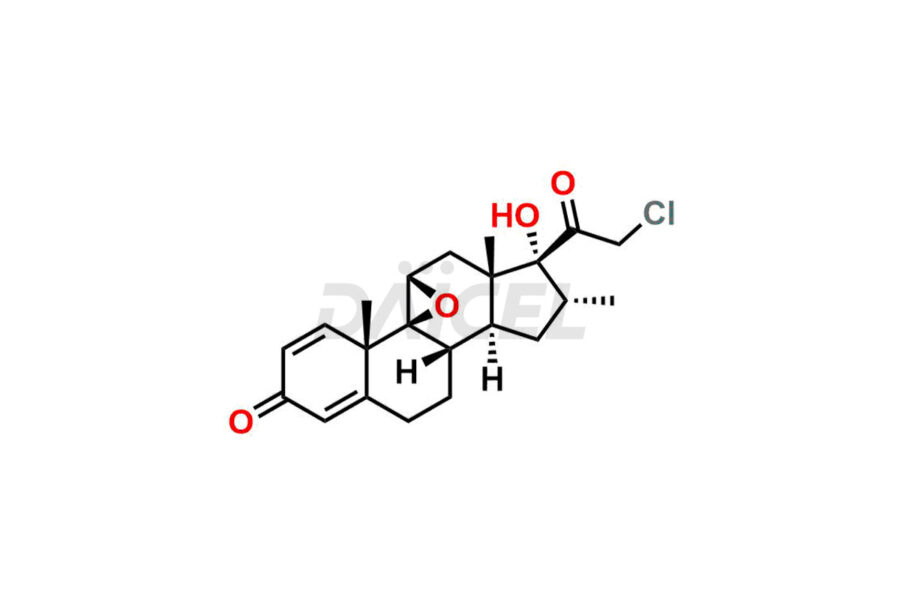
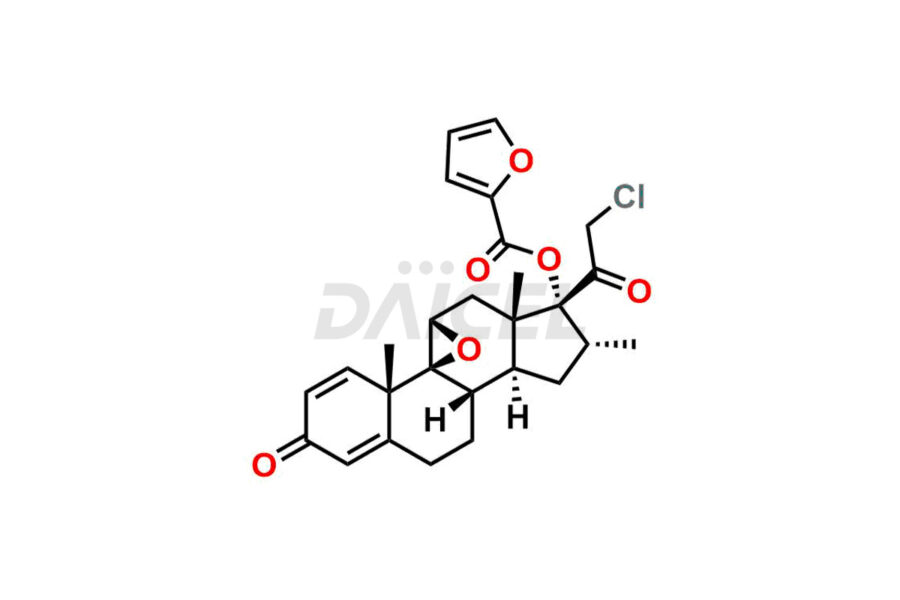
Mometasone Furoate
General Information
Mometasone Furoate Impurities and Mometasone Furoate
For evaluating the purity and safety of Mometasone Furoate, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Mometasone Furoate impurity standards. These impurity standards include crucial compounds such as Mometasone Furoate Anhydrous 8-DM (Impurity L), Mometasone Furoate Anhydrous DMC (Impurity Q), Mometasone Furoate Anhydrous DMCF (Impurity D) and more. Additionally, Daicel Pharma provides worldwide delivery options for Mometasone Furoate impurity standards.
Mometasone Furoate [CAS: 83919-23-7] is a synthetic topical glucocorticoid receptor (GR) agonist. It has vasoconstrictive, anti-inflammatory, and antipruritic properties. It treats asthma, allergic rhinitis, and various skin disorders.
Mometasone Furoate: Use and Commercial Availability
Mometasone Furoate, marketed under brand names like Asmanex HFA, Asmanex Twisthaler, Elocon, Nasonex, Nasonex 24HR allergy, and Sinuva, offers multiple treatment options. It is available as an inhalant for preventing asthma in patients aged four years and older. As a topical ointment, it treats dermatitis and pruritus in patients aged two years and above. The nasal spray form is offered both over-the-counter and by prescription. The OTC nasal spray is for upper respiratory allergic symptoms in patients aged two years and above. The prescription nasal spray is for chronic rhinosinusitis with nasal polyps in patients aged 18 years and above. It prevents seasonal allergic rhinitis in patients aged 12 years and older.
Mometasone Furoate Structure and Mechanism of Action 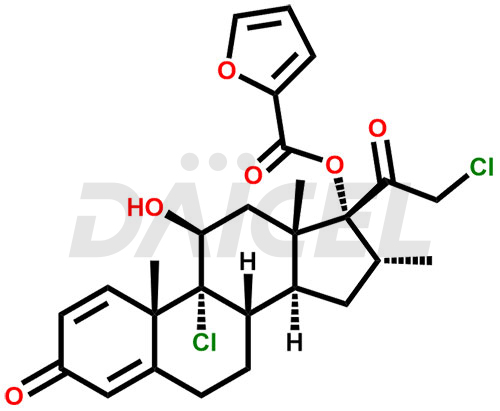
The chemical name of Mometasone Furoate is (11β,16α)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methylpregna-1,4-diene-3,20-dione. Its chemical formula is C27H30Cl2O6, and its molecular weight is approximately 521.4 g/mol.
The precise mechanism of action of Mometasone Furoate is unclear.
Mometasone Furoate Impurities and Synthesis
Like any chemical compound, Mometasone Furoate1 may contain impurities that can impact its quality and safety. Common impurities in Mometasone Furoate formulations can include related substances, residual solvents, and degradation products. Regulatory guidelines provide limits for these impurities to ensure product quality and safety. Manufacturers employ strict quality control measures and analytical techniques to monitor and control impurity levels in Mometasone Furoate.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Mometasone Furoate impurity standards, which include Mometasone Furoate Anhydrous 8-DM (Impurity L), Mometasone Furoate Anhydrous DMC (Impurity Q), and Mometasone Furoate Anhydrous DMCF (Impurity D). Mometasone Furoate impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Mometasone Furoate impurity standards and degradation products. Each delivery has a comprehensive characterization report, ensuring quality and transparency.
References
FAQ's
References
- Shapiro, Elliot L., Aromatic Heterocyclic Esters Of Steroids, Their Preparation And Pharmaceutical Compositions Containing Them, Schering Corp., United States, EP57401B1, August 1, 1984
- Teng, X. W.; Foe, K.; Brown, K. F.; Cutler, D. J.; Davies, N. M., High-performance liquid chromatographic analysis of mometasone furoate and its degradation products. Application to in vitro degradation studies, Journal of Pharmaceutical and Biomedical Analysis, Volume: 26, Issue: 2, Pages: 313-319, 2001
Frequently Asked Questions
How are Mometasone Furoate impurities identified and quantified?
Manufacturers use various analytical techniques, such as chromatography and spectroscopy, to identify and quantify impurities in Mometasone Furoate.
Can impurity levels vary between different Mometasone Furoate products?
Yes, impurity levels can vary between different Mometasone Furoate products based on factors such as manufacturing processes and storage conditions. Manufacturers are responsible for maintaining consistent quality and monitoring impurity levels.
Which solvent helps in analyzing Mometasone Furoate impurities?
Acetonitrile, or DMSO, is solvent when analyzing many impurities in Mometasone Furoate.
What is the recommended storage temperature for Mometasone Furoate impurities?
Mometasone Furoate impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.