Minoxidil
General Information
Minoxidil Impurities and Minoxidil
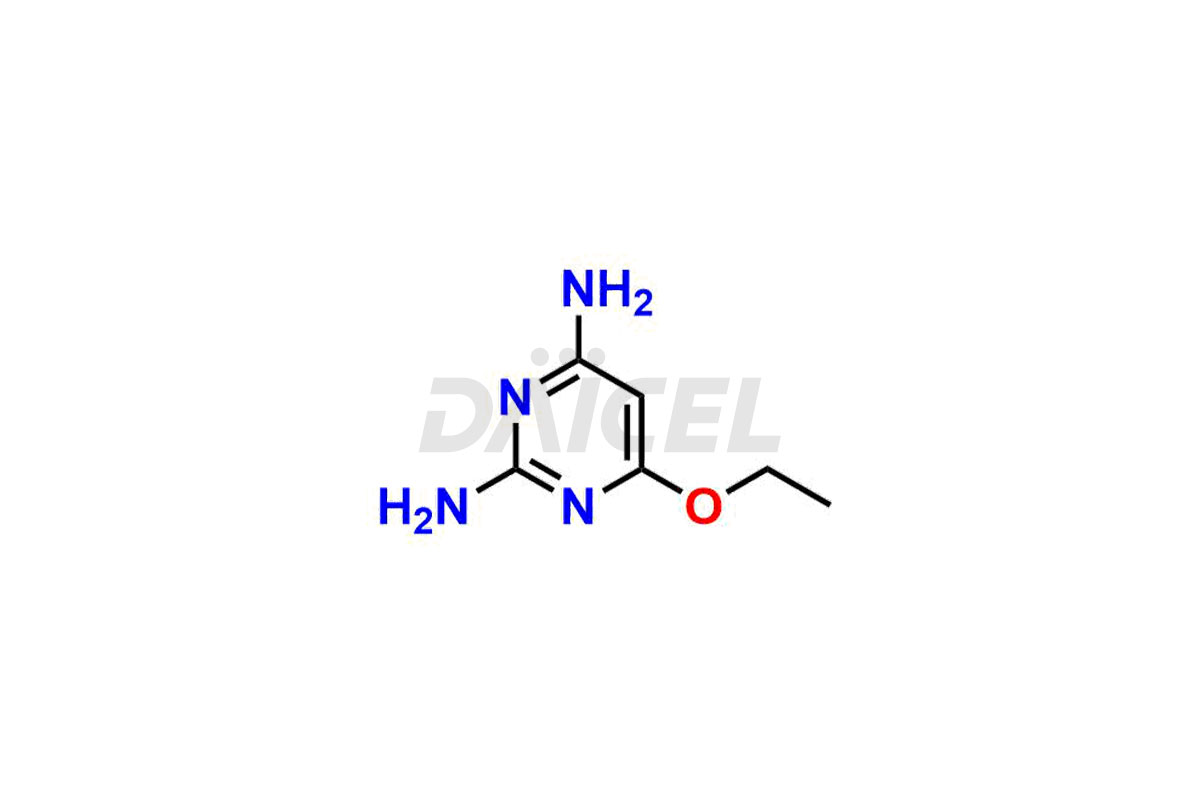
Daicel Pharma is a trusted provider of quality Minoxidil impurity standards such as 2,4-diamino-6-ethoxy pyrimidine etc. These impurities are critical in determining Minoxidil’s quality, stability, and biological safety. Additionally, Daicel Pharma can synthesize Minoxidil impurities according to precise customer specifications while guaranteeing worldwide delivery.
Minoxidil [CAS: 38304-91-5] is an antihypertensive agent used for treating severe hypertension and the topical treatment (hair regrowth) of androgenic alopecia in males and females.
Minoxidil: Use and Commercial Availability 
Minoxidil is most commonly used to treat androgenetic alopecia, also known as male or female pattern baldness, promoting hair growth. It prevents further hair loss. It increases blood flow to the hair follicles, stimulating hair growth in individuals experiencing hair thinning or baldness.
Minoxidil is available under the brand names Loniten, Men’s Rogaine, Women’s Rogaine, Minodyl, Theroxidil, etc., which contains the active ingredient Minoxidil.
Minoxidil Structure and Mechanism of Action
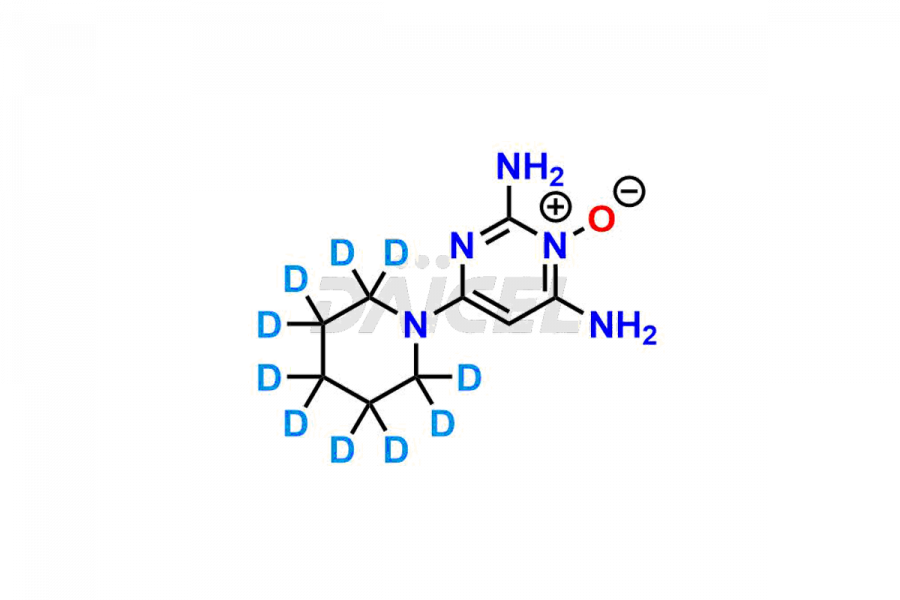
The chemical name of Minoxidil is 2,4-Diamino-6-(1-piperidinyl)-pyrimidine-3-oxide. Its chemical formula is C9H15N5O, and its molecular weight is approximately 209.25 g/mol.
Minoxidil activates extracellular signal-regulated kinase (ERK) and Akt, stimulating human hair growth.
Minoxidil Impurities and Synthesis
Minoxidil impurities can arise during synthesis1 due to the storage or use of specific raw materials and intermediates in manufacturing. These impurities encompass related compounds, degradation products, and process impurities. Stringent quality control measures and analytical methods are crucial to ensure the purity and safety of Minoxidil for patient use.
Daicel Pharma provides a comprehensive Certificate of Analysis (CoA) for Minoxidil impurity standards, such as 2,4-diamino-6-ethoxy pyrimidine. The CoA includes detailed characterization data such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity2. Additionally, upon delivery, a complete 13C-DEPT is also provided. Daicel possesses the technology and expertise to synthesize any unknown Minoxidil impurity or degradation product. We offer Minoxidil-D10, a deuterium-labeled compound of Minoxidil, essential in BA/BE studies.
References
FAQ's
References
- Upjohn Co., Improvements in or relating to Substituted Pyrimidines and the manufacture thereof, GB1167735A, October 22, 1969
- Asmus, P. A.; Landis, J. B.; Grant, M. E.; Havel, H. A., Determination of minoxidil in bulk drug and pharmaceutical formulations by ion-pairing high-performance liquid chromatography, Journal of Pharmaceutical Sciences, Volume: 73, Issue: 9, Pages: 1290-3, 1984
Frequently Asked Questions
How can process-related impurities be detected in Minoxidil?
The rapid, reversed-phase high-performance liquid chromatographic (HPLC) method helps detect and analyze Minoxidil impurities.
How to prevent impurities from forming in Minoxidil during storage?
Minoxidil is stored in a cool, dry place and protected from light and moisture to prevent the formation of impurities.
Which solvent helps in the analysis of Minoxidil impurities?
Methanol is one of the solvents used to analyze many Minoxidil impurities.
What are the temperature conditions required to store Minoxidil impurities?
Minoxidil impurities are stored at a controlled room temperature between 2-8 °C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.