Methyldopa
General Information
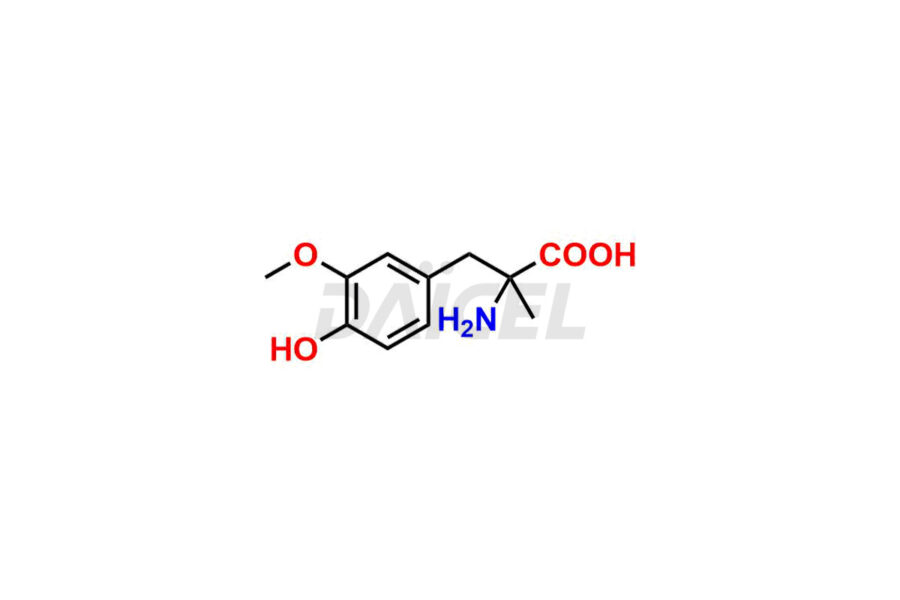
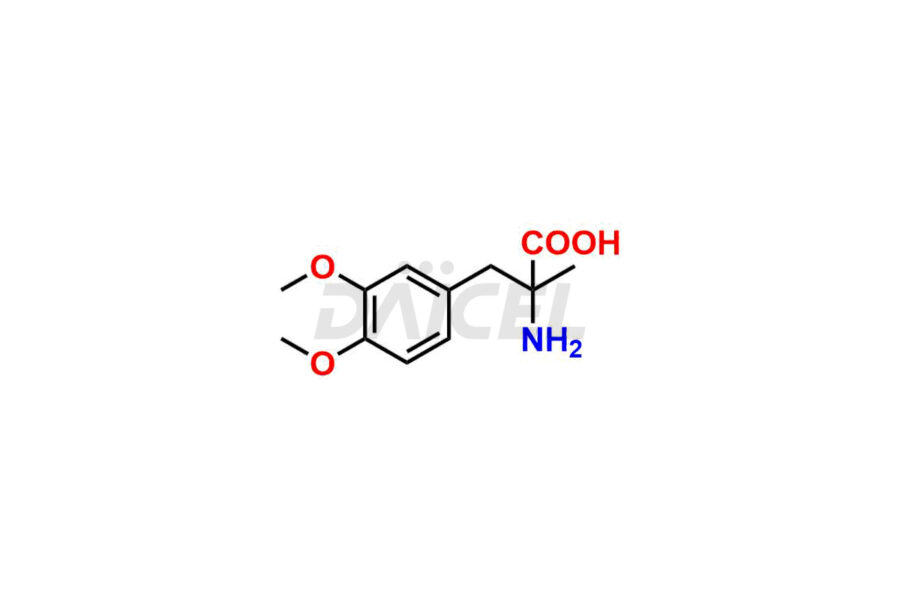
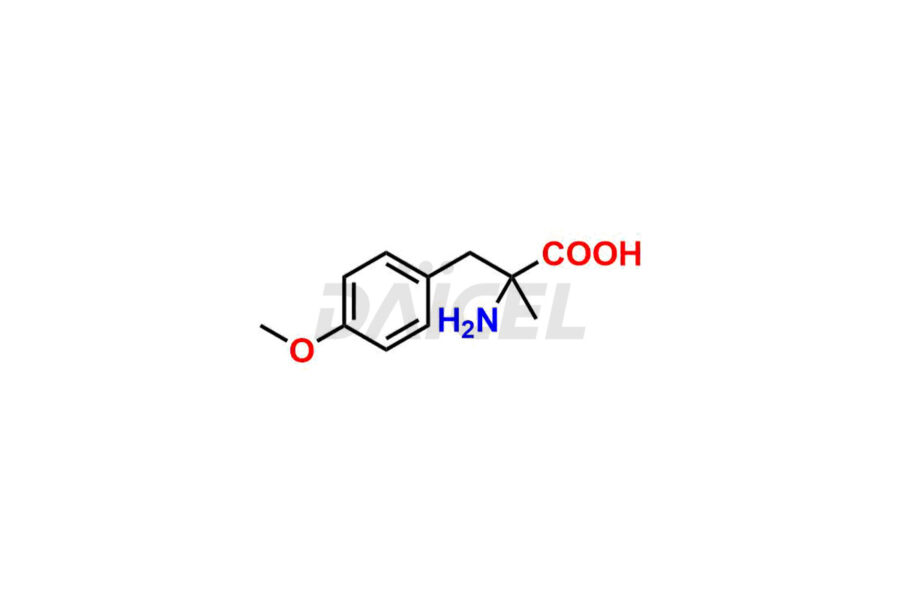
Methyldopa Impurities and Methyldopa
For evaluating the purity and safety of Methyldopa, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Methyldopa impurity standards. These impurity standards include crucial compounds such as 3-O-Methylmethyldopa, Methyldopa related compound C, and Methyldopa related compound-B. Additionally, Daicel Pharma provides worldwide delivery options for Methyldopa impurity standards.
Methyldopa [CAS: 555-30-6] (α-methyldopa) is a centrally active sympatholytic agent for hypertension treatment. It acts as an antihypertensive agent and an alpha-adrenergic agonist, exerting its effects on the peripheral nervous system.
Methyldopa: Use and Commercial Availability
Methyldopa, a centrally acting sympatholytic agent, is for hypertension treatment. It remains in use in developing countries due to its affordability. It belongs to the class of centrally acting antihypertensive drugs. Aldomet, Apo-Methyldopa, Dopamet, and Novomedopa are some brands under which the drug is available.
Methyldopa Structure and Mechanism of Action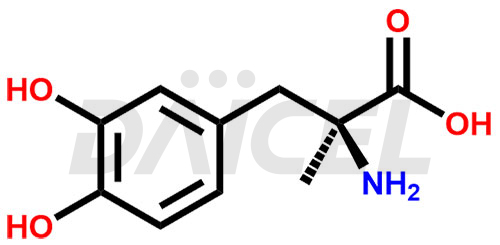
The chemical name of Methyldopa is 3-Hydroxy-α-methyl-L-tyrosine. Its chemical formula is C10H13NO4, and its molecular weight is approximately 211.21 g/mol.
Methyldopa blocks dopa decarboxylase and metabolizes alpha-methylnorepinephrine, lowering arterial pressure by stimulating central inhibitory alpha-methylnorepinephrine.
Methyldopa Impurities and Synthesis
Methyldopa, a centrally acting sympatholytic agent, may contain impurities that can impact its purity and efficacy. Related substances, residual solvents, and degradation products are some of the impurities. Strict quality control measures are necessary during manufacturing1 and storage of Methyldopa to minimize impurity levels and ensure its safety and effectiveness. Monitoring and controlling impurities in Methyldopa is essential to maintain its therapeutic benefits in treating hypertension and other conditions.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Methyldopa impurity standards, which include 3-O-Methylmethyldopa, Methyldopa related compound C, and Methyldopa related compound-B. The Methyldopa impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through various techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Methyldopa impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
Frequently Asked Questions
Can the presence of Methyldopa impurities lead to batch rejections?
The presence of impurities in Methyldopa beyond the acceptable limits can lead to batch rejections. Quality control measures are in place to ensure that the drug meets the predefined specifications and impurity limits. The batch may be rejected If the impurity levels exceeds the specified criteria.
Are Methyldopa impurities monitored throughout the drug's shelf life?
Yes, impurities in Methyldopa are monitored throughout its shelf life. Stability studies are conducted to assess the drug's impurity profile over time, particularly under different storage conditions. These studies help determine the drug's shelf life and ensure the impurity levels remain within acceptable limits.
Can Methyldopa impurities affect the drug's therapeutic response?
Yes, impurities in Methyldopa can potentially impact its therapeutic response. Depending on their nature and concentration, impurities may interfere with the drug's mechanism of action, alter its pharmacokinetics, or introduce unwanted effects that can affect the desired therapeutic outcomes.
What is the recommended storage temperature for Methyldopa impurities?
Methyldopa impurities should be stored at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.