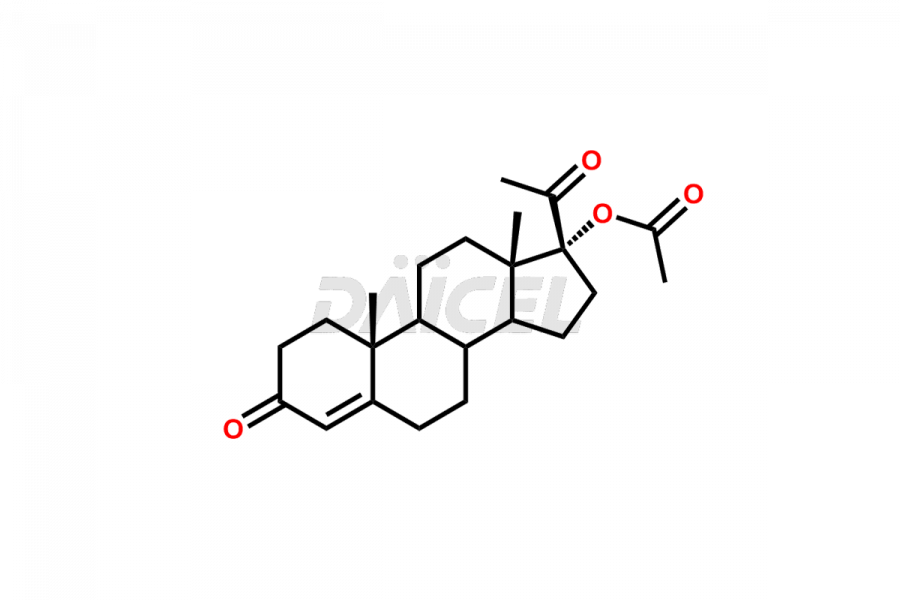
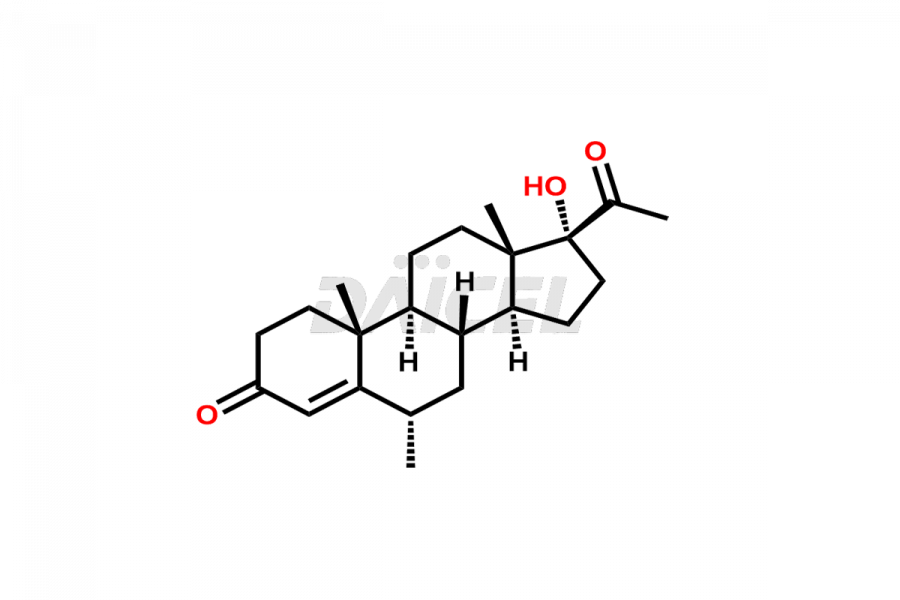
Medroxy progesterone
General Information
Medroxyprogesterone Impurities and Medroxyprogesterone
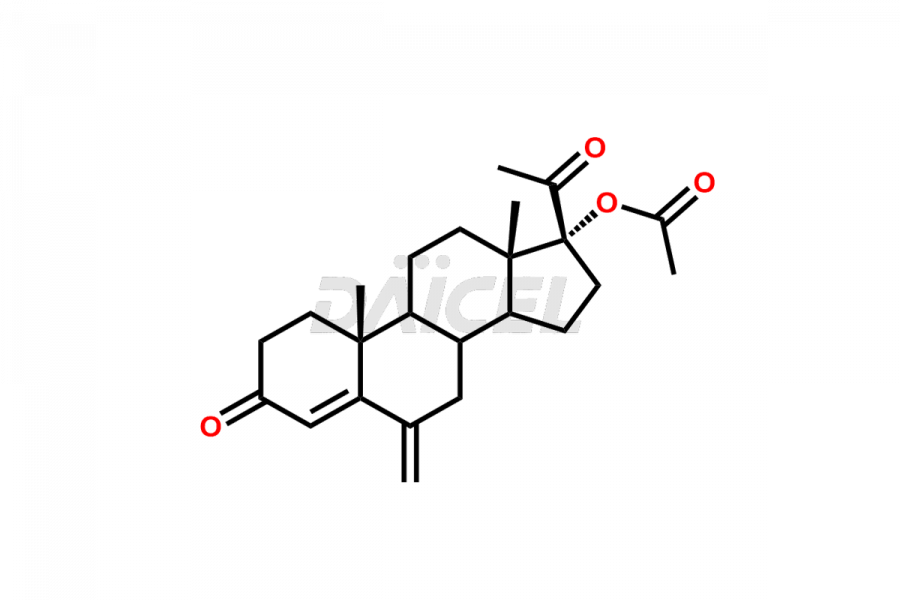
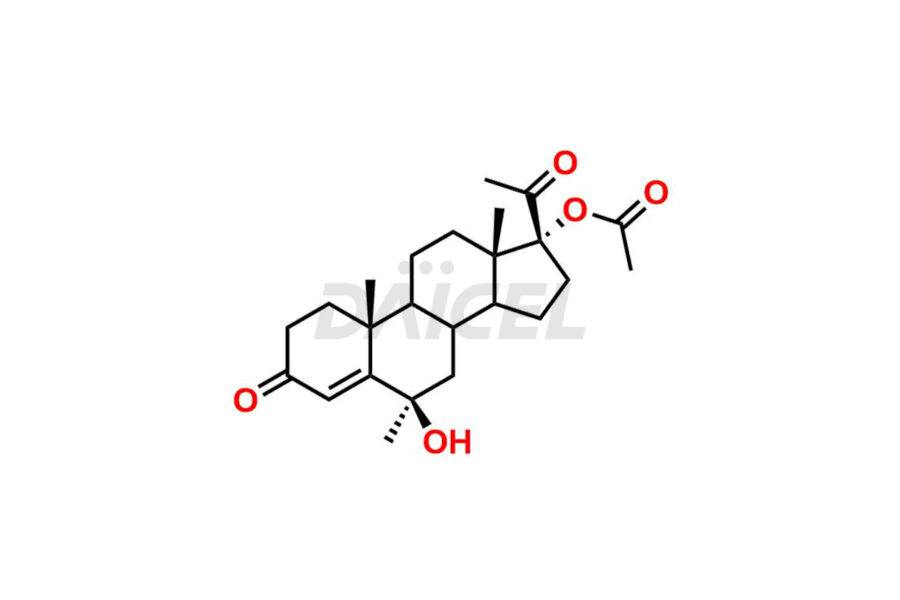
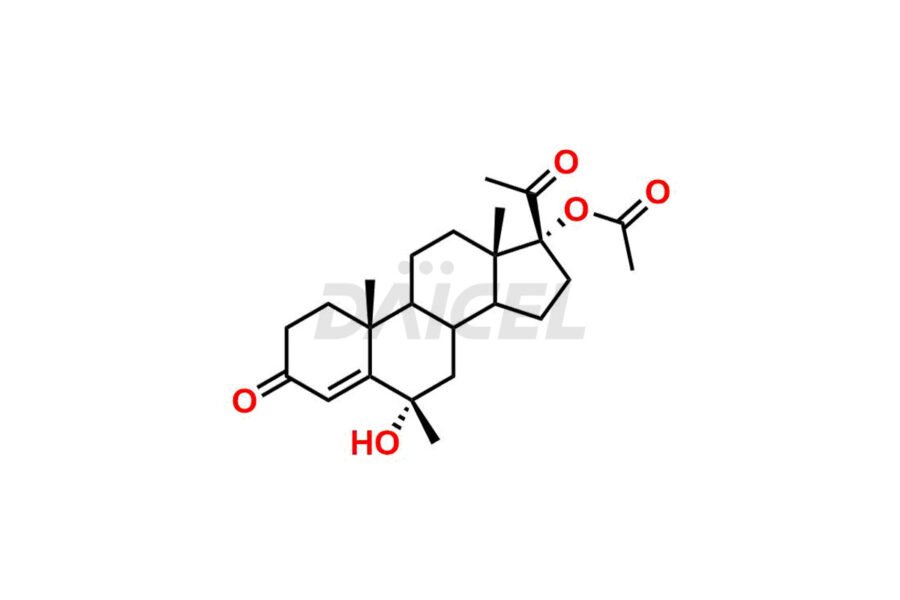
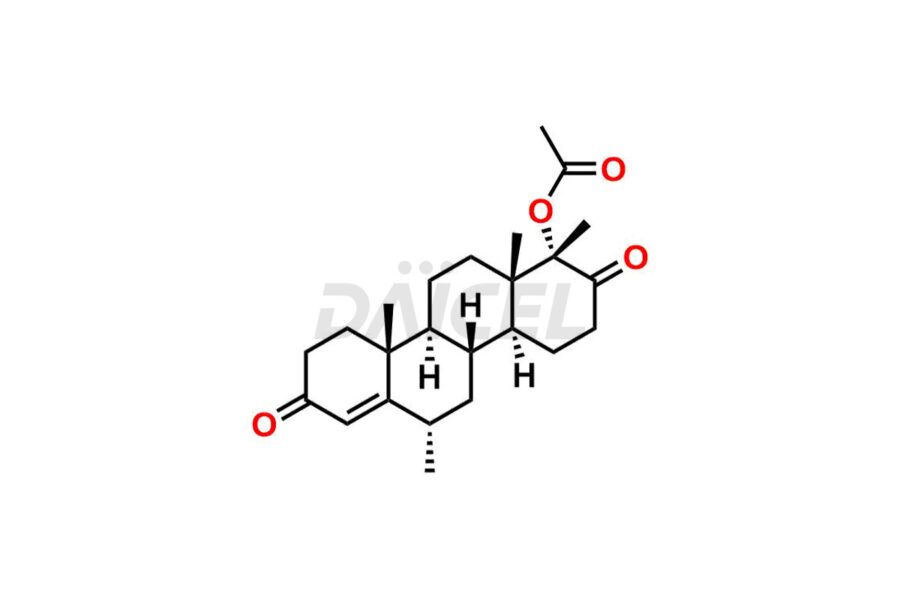
For evaluating the purity and safety of Medroxyprogesterone, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Medroxyprogesterone impurity standards. These impurity standards include crucial compounds such as 6-methylene acetoxy progesterone (Impurity E- Ph Eur), D-Homo analogous Medroxy Progesterone (Imp C Ph. Eur), 6-β -hydroxy Medroxy Progesterone, 6α-Hydroxy Medroxy Progesterone 17-Acetate, 4,5-dihydro analog of Medroxyprogesterone (Impurity F), and more. Additionally, Daicel Pharma provides worldwide delivery options for Medroxyprogesterone impurity standards.
Medroxyprogesterone [CAS: 520-85-4] is a synthetic derivative of progesterone administered as Medroxyprogesterone acetate. It possesses antiestrogenic activity and acts as a progestin. Additionally, Medroxyprogesterone functions as a contraceptive drug and a synthetic oral contraceptive.
Medroxyprogesterone: Use and Commercial Availability
Medroxyprogesterone (MPA) is available under different brand names such as Depo-Provera, Provera, Curretab, Amen, and Cycrin. It is a versatile medication with various therapeutic applications. Medroxyprogesterone acetate is a contraceptive to prevent pregnancy in women of reproductive age. It treats secondary amenorrhea and abnormal uterine bleeding and prevents Endometrial hyperplasia. It also used as an estrus regulator in veterinary practice.
Medroxyprogesterone Structure and Mechanism of Action 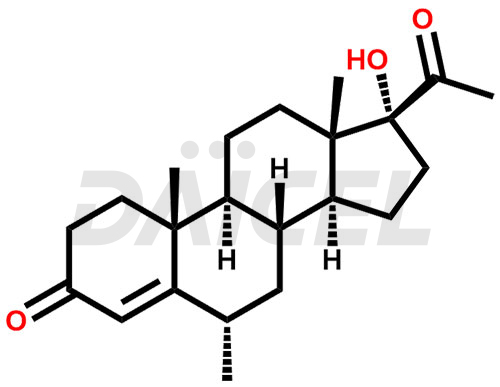
The chemical name of Medroxyprogesterone is (6α)-17-Hydroxy-6-methylpregn-4-ene-3,20-dione. Its chemical formula is C22H32O3, and its molecular weight is approximately 344.5 g/mol.
Medroxyprogesterone blocks gonadotropin production. It prevents follicular maturation and ovulation.
Medroxyprogesterone Impurities and Synthesis
Regular testing and monitoring of Medroxyprogesterone impurities is essential to ensure its purity and quality. Medroxyprogesterone, a synthetic progesterone derivative, may contain impurities from manufacturing1,2 or degradation. They can include related substances, degradation products, or residual solvents used during production. Compliance with regulatory standards ensures acceptable impurity levels in Medroxyprogesterone formulations, guaranteeing product safety and efficacy. Stringent quality control measures and analytical techniques help identify and quantify impurities in Medroxyprogesterone samples. Their continuous monitoring is necessary to maintain its effectiveness and integrity throughout its shelf life.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Medroxyprogesterone impurity standards, which include 6-methylene acetoxy progesterone (Impurity E- Ph Eur), D-Homo analogous Medroxyprogesterone (Imp C Ph. Eur), 6-β -hydroxy Medroxy Progesterone, 6α-Hydroxy Medroxy Progesterone 17-Acetate, 4,5-dihydro analog of Medroxyprogesterone (Impurity F), and more. Our Medroxyprogesterone impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis3. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Medroxyprogesterone impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Miramontes, Luis; Romero, Miguel A.; Fritsche, O., Preparation of 6-methyl steroids of the pregnane series, G.D. Searle and Co., US2878247A, March 17, 1959
- Cavina, G.; Valvo, L.; Alimenti, R., Quantitative analysis and purity evaluation of medroxyprogesterone acetate by HPLC, Journal of Pharmaceutical and Biomedical Analysis Volume: 3, Issue: 6, Pages: 535-46, 1985
Frequently Asked Questions
Can Medroxyprogesterone impurities be present in different ratios depending on the manufacturing process?
Yes, impurity ratios in Medroxyprogesterone can vary depending on the manufacturing process employed by different pharmaceutical manufacturers.
Can Medroxyprogesterone impurities affect the dissolution rate of the drug?
Some impurities in Medroxyprogesterone can impact the dissolution rate of the drug, which may affect its bioavailability and therapeutic effectiveness.
Are there specific stability-indicating methods to monitor Medroxyprogesterone impurities?
Stability-indicating methods, such as forced degradation studies, are employed to monitor impurities in Medroxyprogesterone and assess their impact on product stability.
What is the recommended storage temperature for Medroxyprogesterone impurities?
Medroxyprogesterone impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.