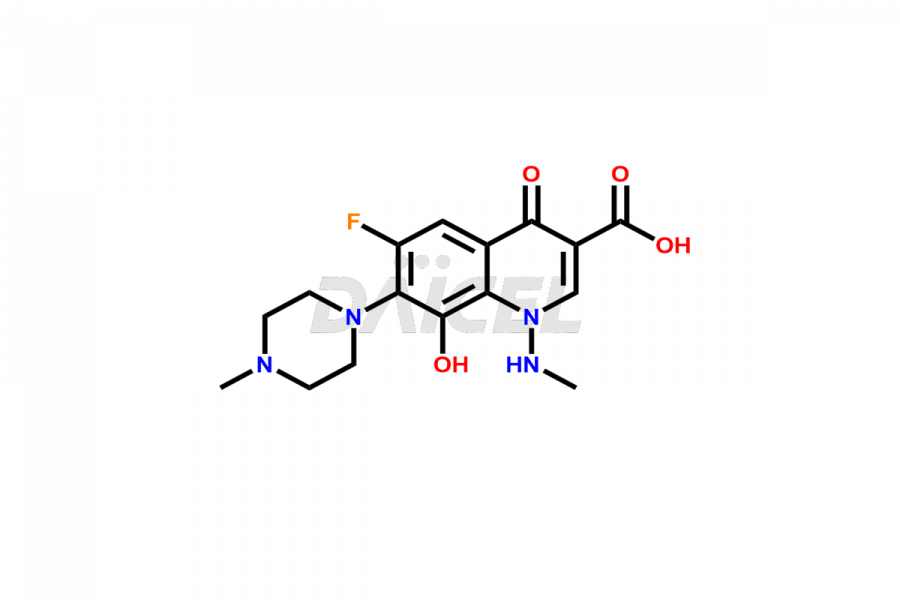
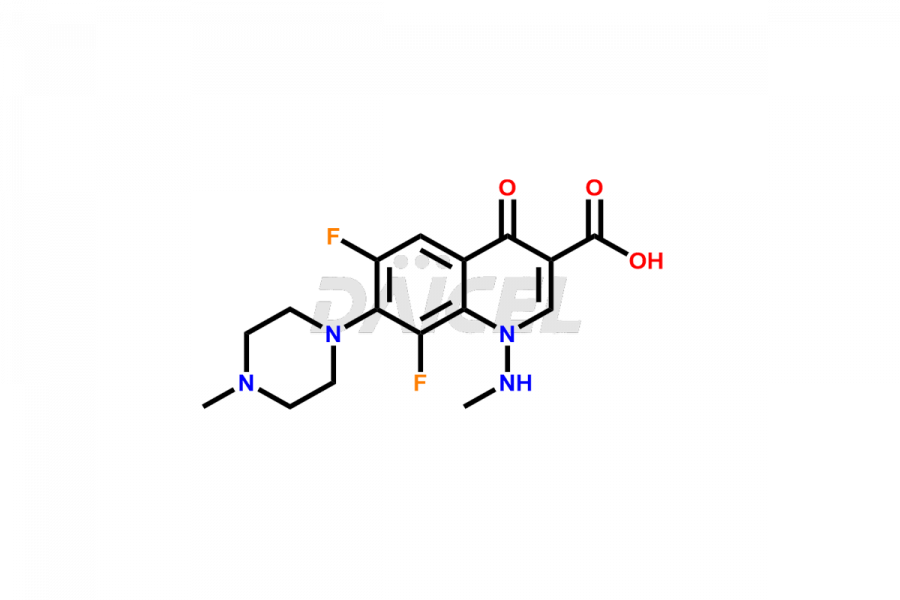
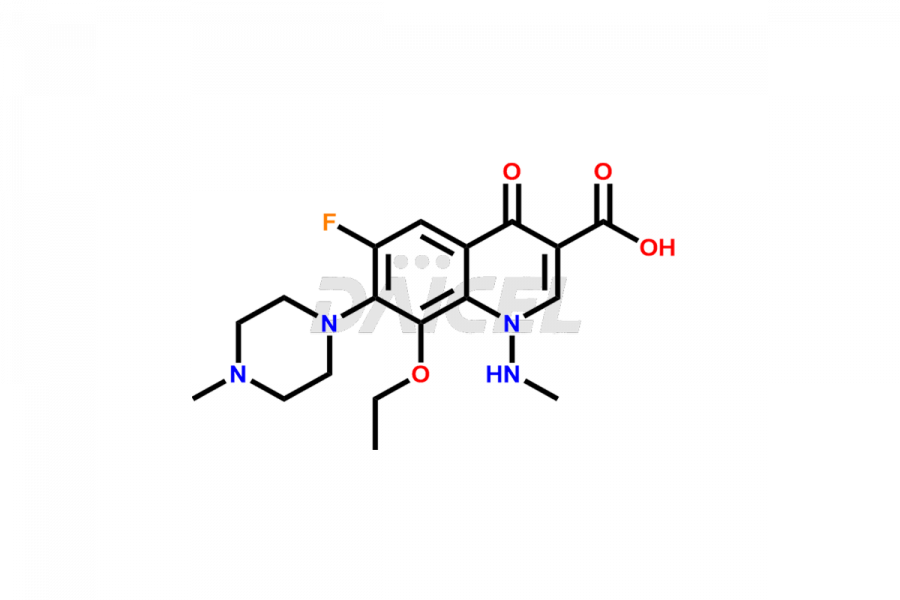
Marbofloxacin
General Information
Marbofloxacin Impurities and Marbofloxacin
For evaluating the purity and safety of Marbofloxacin, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Marbofloxacin impurity standards. These impurity standards include crucial compounds such as Marbofloxacin EP Impurity A, Marbofloxacin EP Impurity B, Marbofloxacin EP Impurity D, Marbofloxacin Impurity C, and Marbofloxacin Impurity E. Additionally, Daicel Pharma provides worldwide delivery options for Marbofloxacin impurity standards.
Marbofloxacin [CAS: 115550-35-1] is a carboxylic acid compound classified as a third-generation fluoroquinolone antibiotic. It is used in veterinary medicine. It has antimicrobial activity and treats dermatological, respiratory, and urinary tract infections from Gram-positive and Gram-negative bacteria.
Marbofloxacin: Use and Commercial Availability
Marbofloxacin, marketed as Marbocyl, Zeniquin, etc., is a newer fluoroquinolone antibiotic known for its broad spectrum of activity against various bacteria and mycoplasmas. It exhibits bactericidal properties. Marbofloxacin treats bone infections, urinary tract infections, skin infections, and infections caused by intracellular organisms.
Marbofloxacin Structure and Mechanism of Action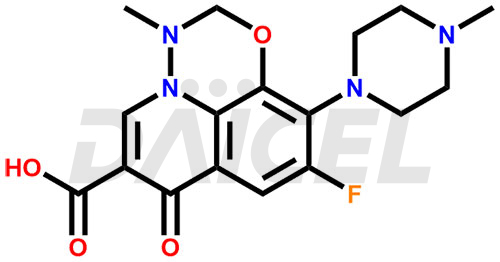
The chemical name of Marbofloxacin is 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,3,4]oxadiazino[6,5,4-ij]quinoline-6-carboxylic acid. Its chemical formula is C17H19FN4O4, and its molecular weight is approximately 362.4 g/mol.
Marbofloxacin inhibits DNA gyrase and topoisomerase IV, enzymes crucial for DNA replication and repairs.
Marbofloxacin Impurities and Synthesis
Regular testing and monitoring of Marbofloxacin impurities are essential to maintain the drug’s purity and integrity. Marbofloxacin, a fluoroquinolone antibiotic, may contain impurities originating from manufacturing1 or degradation. They can include related substances, degradation products, or residual solvents used during production. Strict adherence to regulatory standards ensures permissible impurity levels in Marbofloxacin formulations, guaranteeing product safety and efficacy. Rigorous quality control measures and analytical techniques help identify and quantify impurities in Marbofloxacin samples. This monitoring ensures that Marbofloxacin maintains its effectiveness and quality throughout its shelf life.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Marbofloxacin impurity standards, which include Marbofloxacin EP Impurity A, Marbofloxacin EP Impurity B, Marbofloxacin EP Impurity D, Marbofloxacin Impurity C, and Marbofloxacin Impurity E. Our Marbofloxacin impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Marbofloxacin impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Aoki, Masahiro; Kamata, Miyako; Otsuka, Tatsuo; Shimma, Nobuo; Yokose, Kazuteru, Pyrido[3,2,1-ij]-1,3,4-benzoxadiazine derivatives, process for their preparation, resulting pharmaceutical preparations and intermediates for use in the process, Hoffmann-La Roche, F., und Co. A.-G., Switzerland, EP259804B1, November 18, 1993
- Garcia, M. A.; Solans, C.; Aramayona, J. J.; Rueda, S.; Bregante, M. A., Determination of marbofloxacin in plasma samples by high-performance liquid chromatography using fluorescence detection, Journal of Chromatography B: Biomedical Sciences and Applications Volume: 729, Issue: 1 + 2, Pages: 157-161, 1999
Frequently Asked Questions
Can Marbofloxacin impurities impact its shelf life?
Some impurities in Marbofloxacin can contribute to degradation, affecting the drug's stability and shelf life. Proper storage conditions maintain product quality.
Can Marbofloxacin impurities be responsible for variations in therapeutic efficacy?
Marbofloxacin impurities within acceptable limits may not affect its therapeutic efficacy. The active ingredient is primarily responsible for the drug's therapeutic effects.
Can Marbofloxacin impurities undergo further chemical reactions within the body?
Impurities in Marbofloxacin can potentially undergo chemical reactions within the body, but their occurrence and impact depend on their specificity and reactivity.
What is the recommended storage temperature for Marbofloxacin impurities?
Marbofloxacin impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.