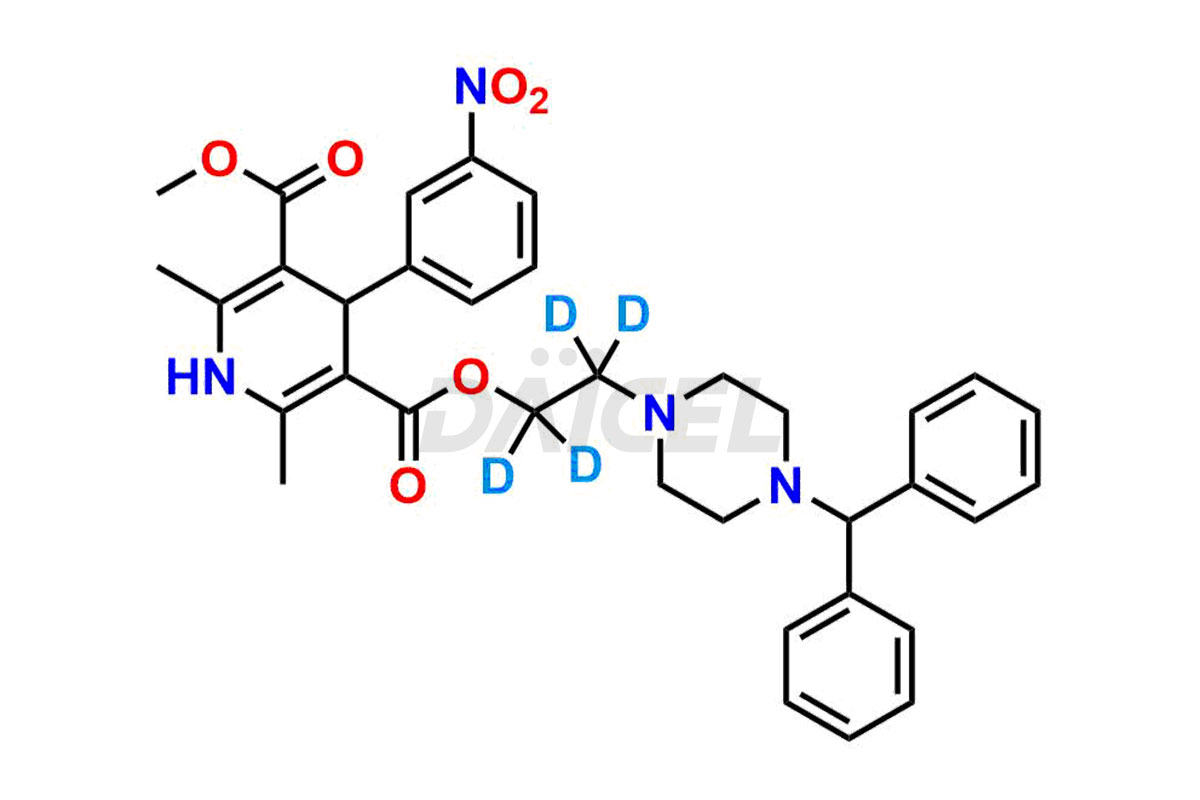
Manidipine
General Information
Manidipine Impurities and Manidipine
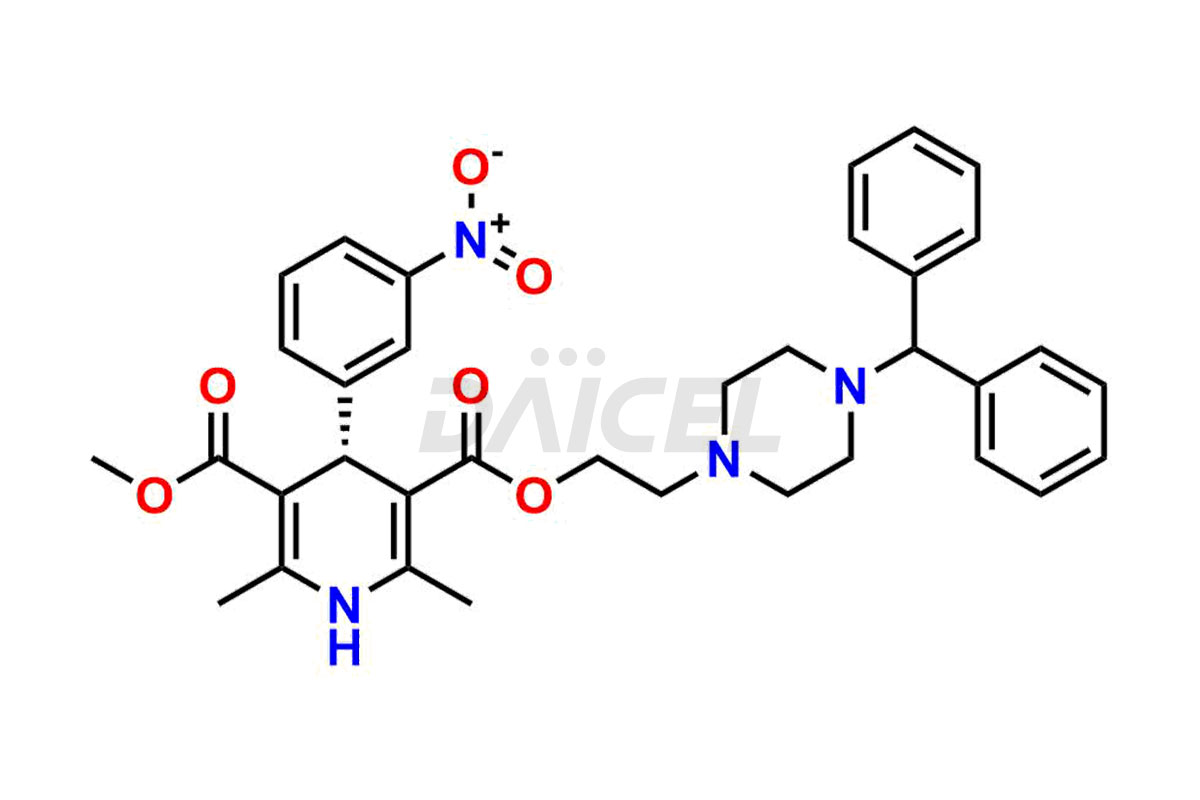
For evaluating the purity and safety of Manidipine, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Manidipine impurity standards. These impurity standards include crucial compounds such as (R)-Manidipine and (S)-Manidipine. Additionally, Daicel Pharma provides worldwide delivery options for Manidipine impurity standards.
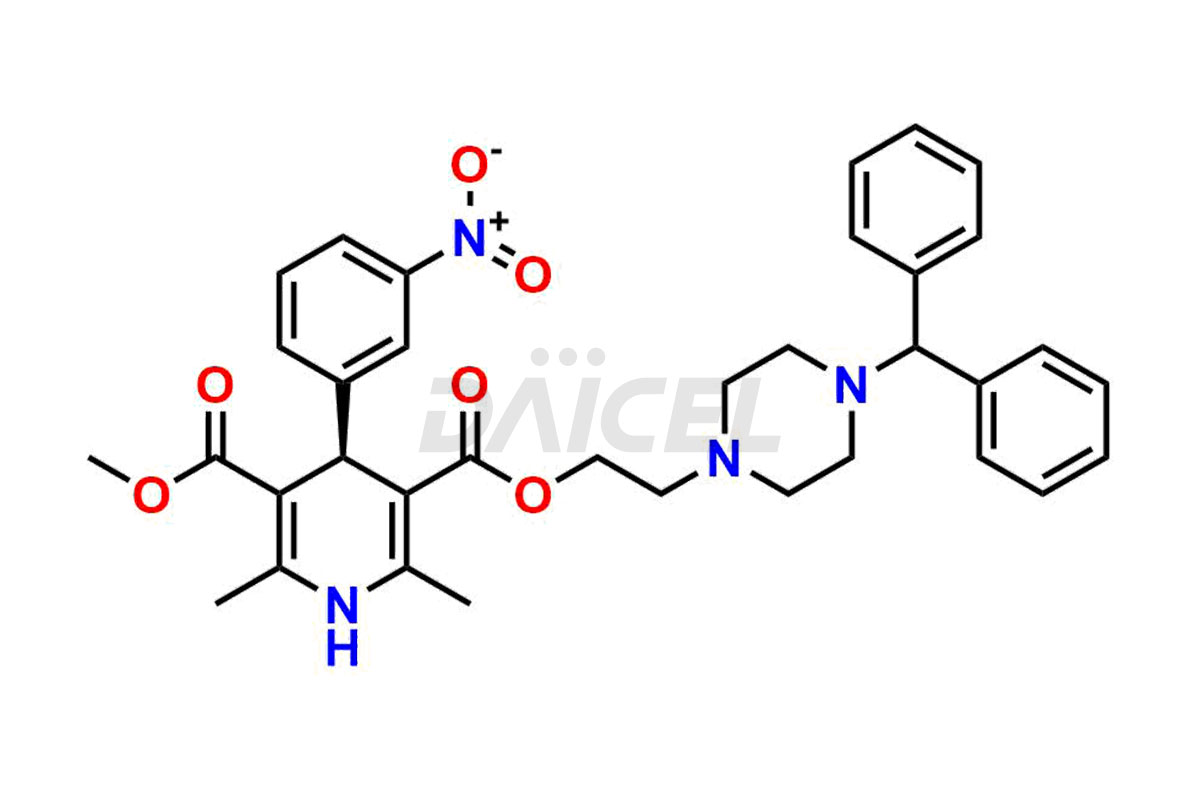
Manidipine [CAS: 89226-50-6] is a diarylmethane compound. It belongs to the dihydropyridine class of calcium channel blockers and is an antihypertensive medication for treating high blood pressure.
Manidipine: Use and Commercial Availability
Manidipine has demonstrated efficacy as an antihypertensive agent in adult and elderly patients with mild or moderate essential hypertension. As a dihydropyridine calcium antagonist, Manidipine reduces blood pressure in hypertensive patients. Manidipine is available globally under various brand names, including Iperten, Presidin, Artedil, Caldine, etc.
Manidipine Structure and Mechanism of Action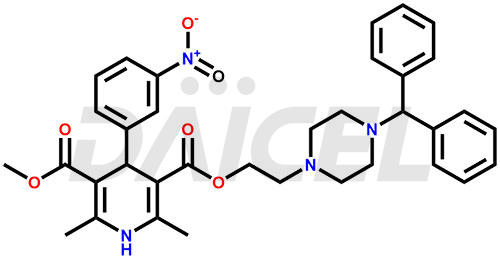
The chemical name of Manidipine is 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 3,5-Pyridinedicarboxylic acid 3-[2-[4-(diphenylmethyl)-1-piperazinyl]ethyl] 5-methyl ester. Its chemical formula is C35H38N4O6, and its molecular weight is approximately 610.7 g/mol.
Manidipine inhibits voltage-dependent calcium currents in smooth muscle cells and causes systemic vasodilation.
Manidipine Impurities and Synthesis
Manidipine, an antihypertensive medication, may contain impurities from various sources, such as manufacturing1 or degradation over time. They can include related substances, isomers, degradation products, and residual solvents used during manufacturing. Stringent quality control measures minimize impurity levels and adhere to regulatory standards to ensure product quality and safety. Regular testing and monitoring of Manidipine samples are conducted to maintain the drug’s purity and efficacy throughout its shelf life.
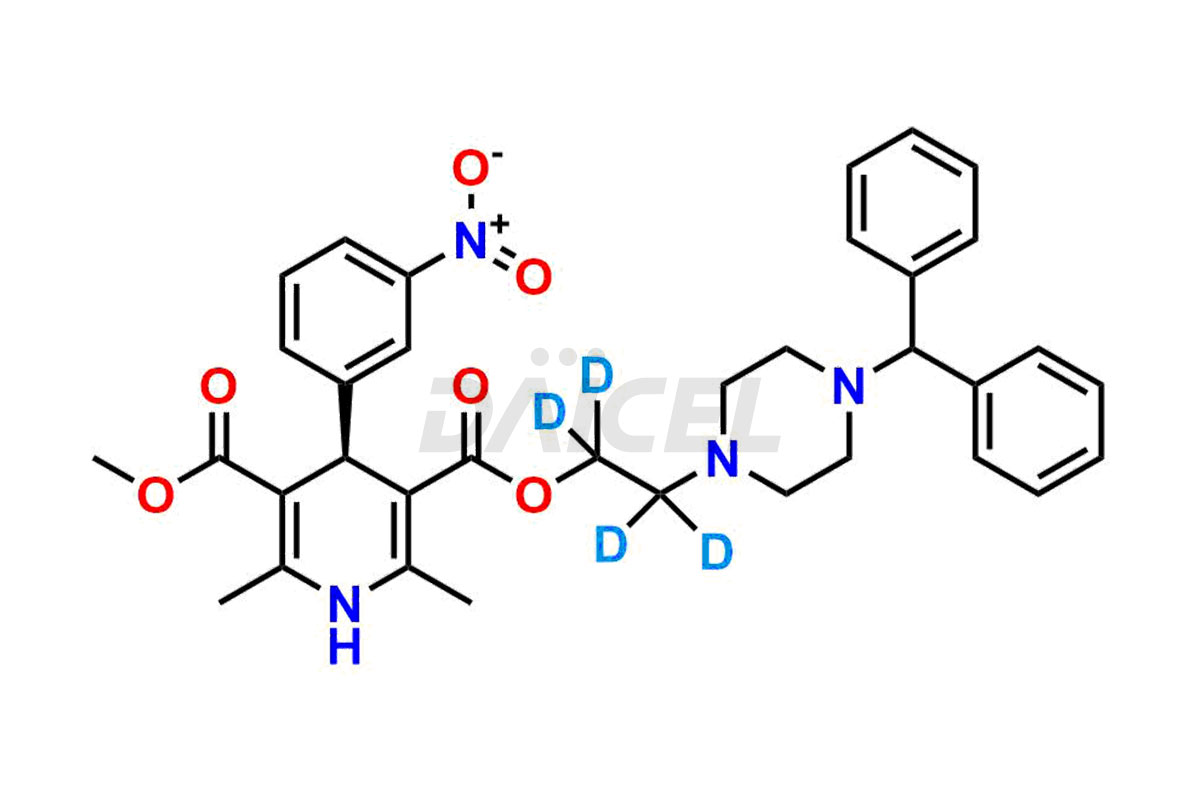
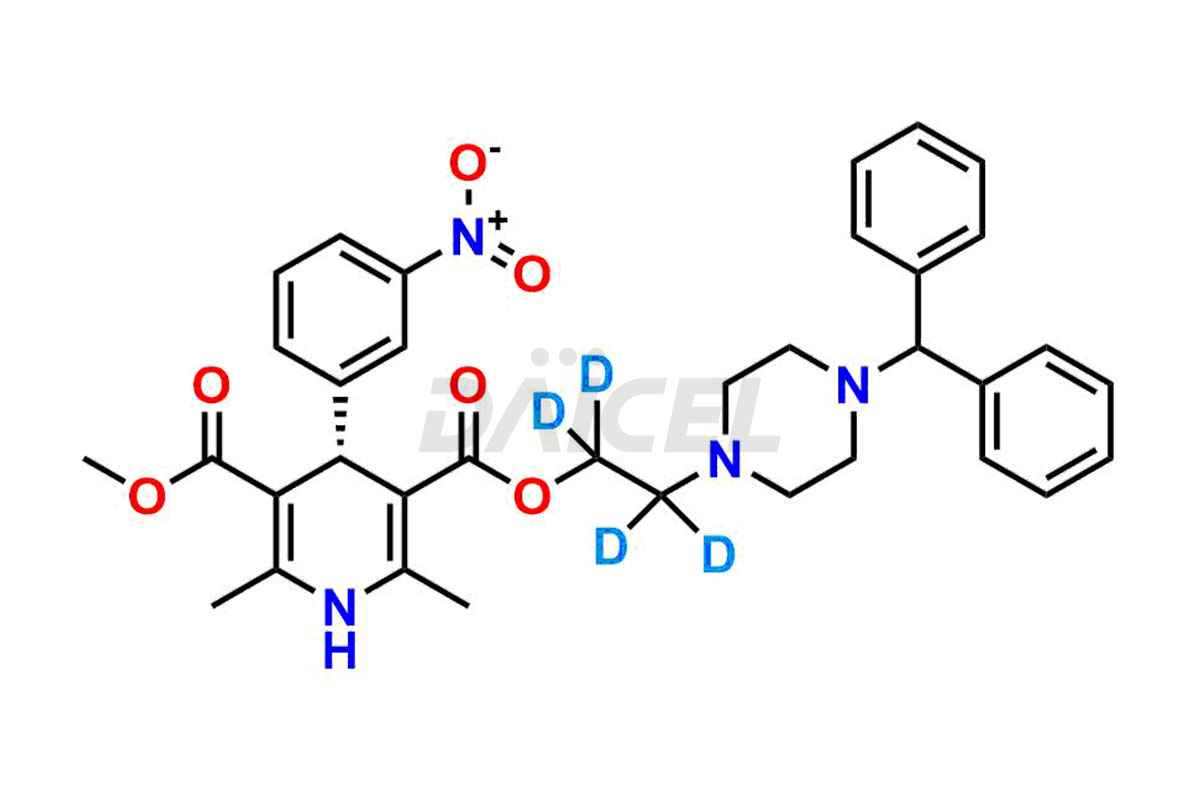
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Manidipine impurity standards, which include (R)-Manidipine and (S)-Manidipine. In addition, we offer deuterium-labeled Manidipine compounds, (R)-Manidipine-D4, (S)-Manidipine-D4, and rac-Manidipine-D4, which are essential for conducting bioanalytical research and BA/BE studies. Our Manidipine impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques,1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Manidipine impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Meguro, Kanji; Nagaoka, Akinobu, Dihydropyridine Derivatives, Their Production and Use, Takeda Chemical Industries, Ltd., Japan, EP94159B1, March 14, 1990
- Miyabayashi, Tetsushirou; Yamashita, Kenji; Aoki, Isamu; Motohashi, Michio; Yashiki, Takatsuka; Yatani, Kozo, Determination of manidipine and its pyridine metabolite in human serum by high-performance liquid chromatography with ultraviolet detection and column switching, Journal of Chromatography, Biomedical Applications,Volume: 494, Pages: 209-17, 1989
Frequently Asked Questions
How are Manidipine impurities identified and characterized?
Impurities in Manidipine are identified and characterized using advanced analytical techniques such as chromatography, mass spectrometry, and spectroscopy.
How often are impurity profiles in Manidipine reviewed and updated?
Impurity profiles in Manidipine are periodically reviewed and updated based on new scientific knowledge and regulatory requirements.
Which solvent helps in analyzing Manidipine impurities?
Methanol is the solvent used when analyzing most impurities in Manidipine.
What is the recommended storage temperature for Manidipine impurities?
Manidipine impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.