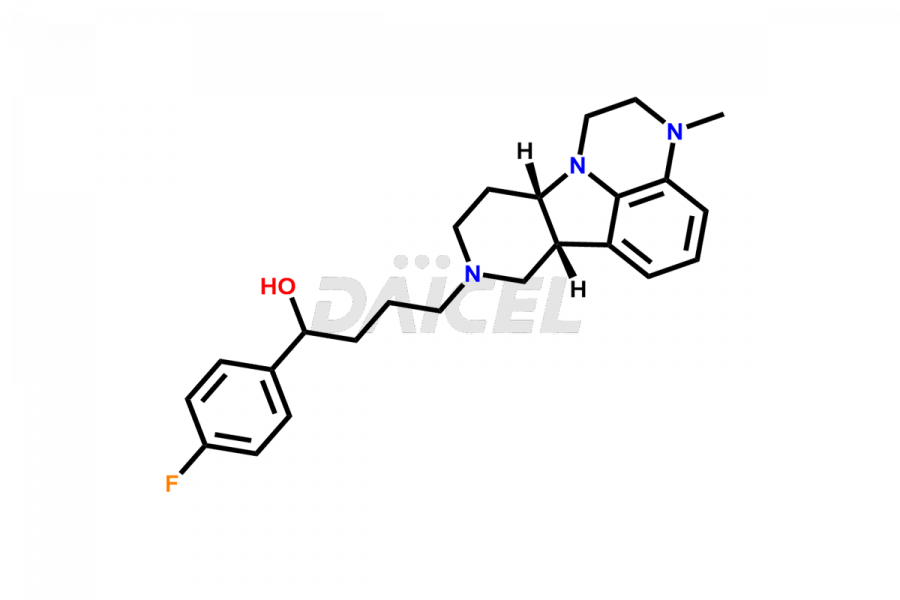
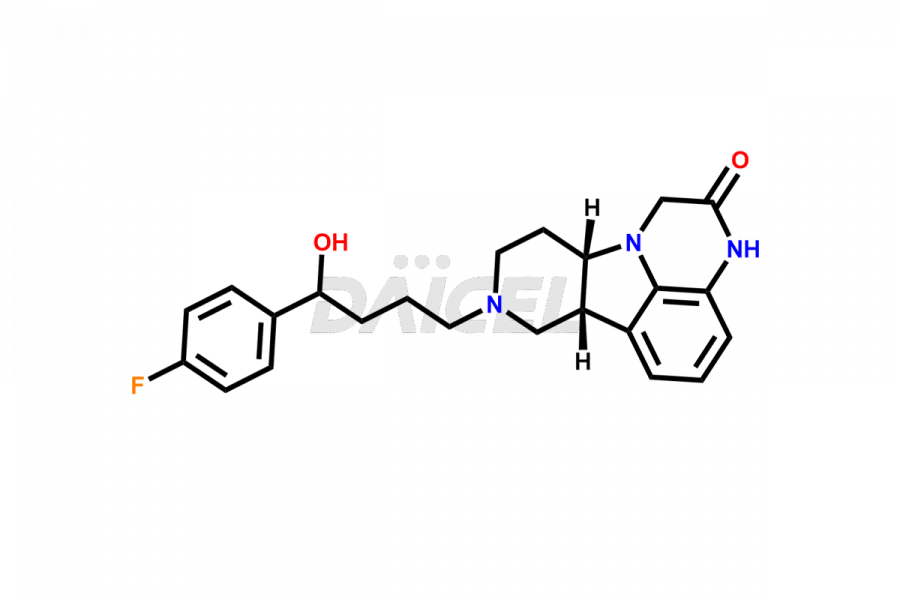
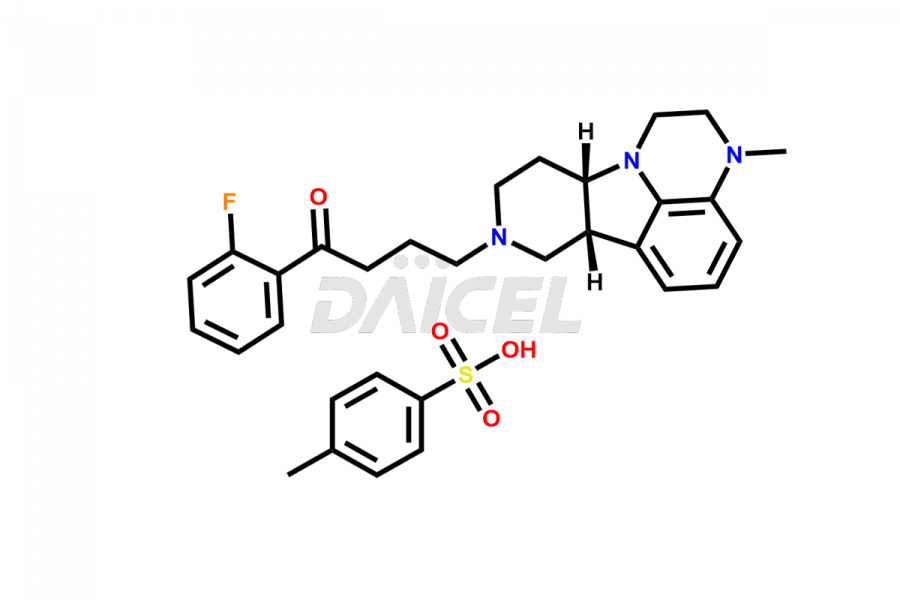
Lumateperone
General Information
Lumateperone Impurities and Lumateperone
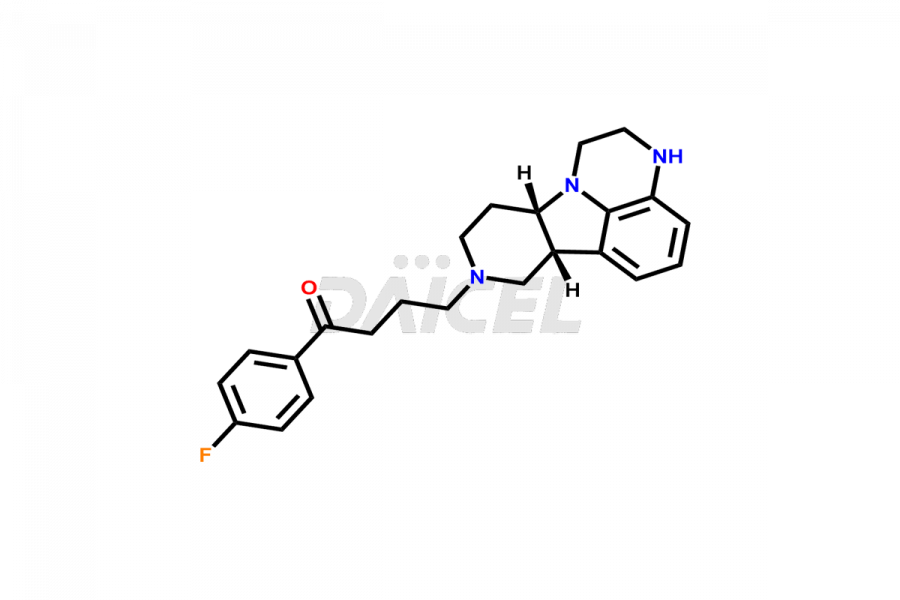
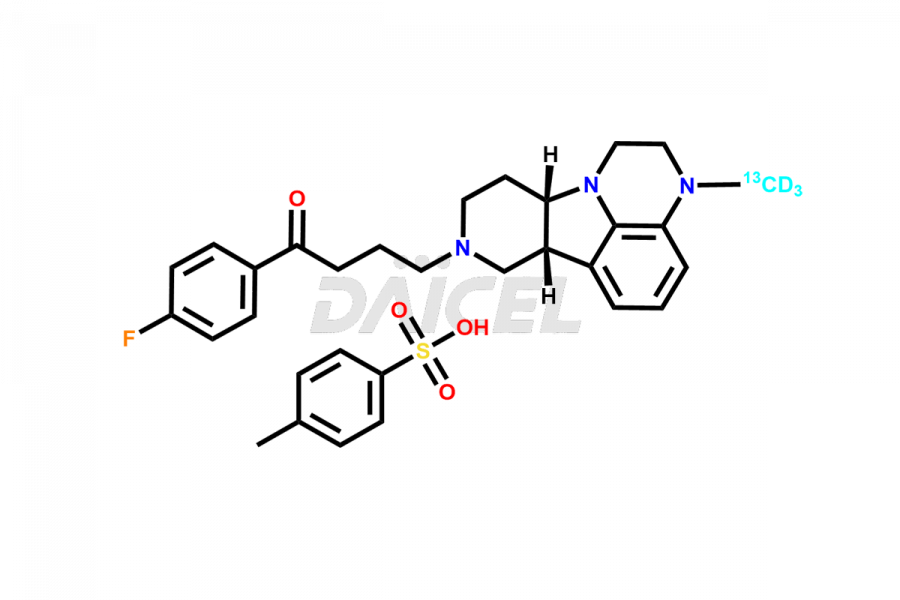
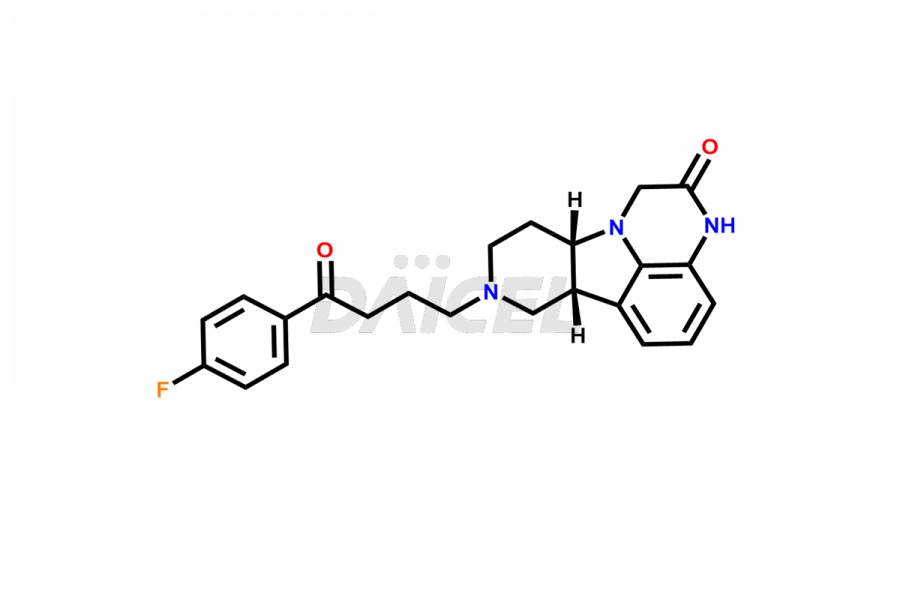
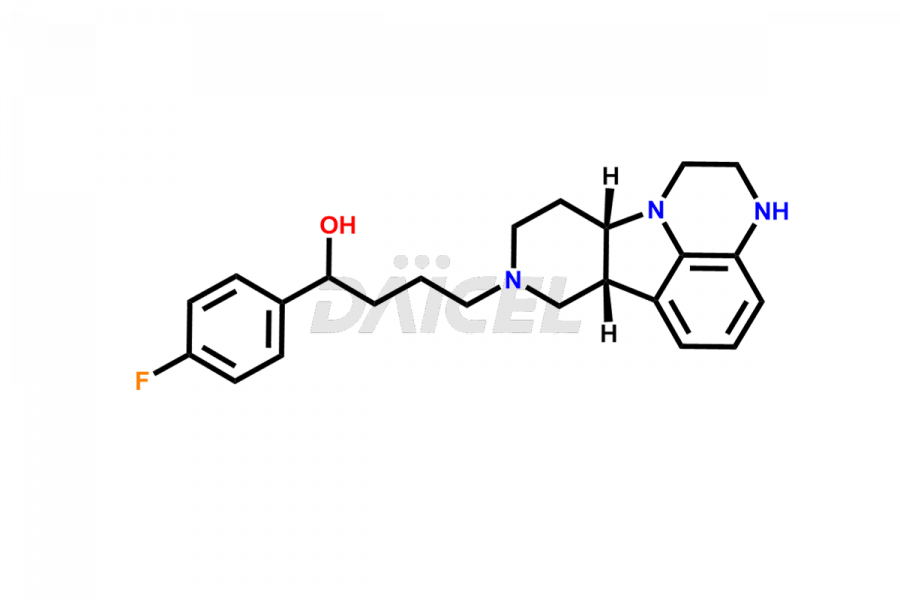
Daicel Pharma is a reliable source for synthesizing high-quality Lumateperone impurities, specifically Desmethyl Lumateperone, Lumateperone Metabolite 308, Lumateperone Metabolite 565, Lumateperone metabolite M131, Lumateperone Metabolite M309, Lumateperone Tosylate, and Ortho Isomer of Lumateperone (Tosylate salt). These impurities help assess the quality, stability, and safety of the active pharmaceutical ingredient, Lumateperone. Daicel Pharma also offers custom synthesis of Lumateperone impurities, which can be shipped worldwide.
Lumateperone [CAS: 313368-91-1] is an atypical antipsychotic medication treating schizophrenia. It belongs to the second generation of antipsychotic drugs and selectively and simultaneously modulates serotonin, dopamine, and glutamate levels in the human brain.
Lumateperone: Use and Commercial Availability
Lumateperone is a medicine to treat schizophrenia in adults and depressive episodes associated with bipolar disorder (known as bipolar depression) in adults, either alone or in combination with lithium or valproate. It is available under the brand name Caplyta. Lumateperone is a second-generation atypical antipsychotic drug. It is unique in its mechanism of action to manage and treat various neuropsychiatric disorders.
Lumateperone Structure and Mechanism of Action 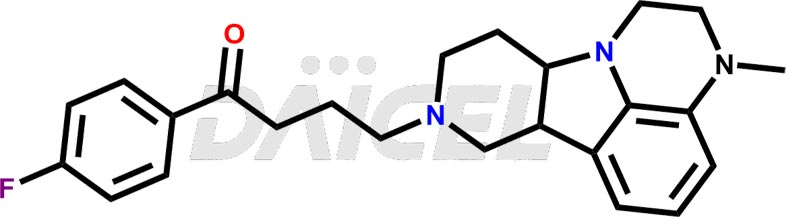
The chemical name of Lumateperone is 1-(4-Fluorophenyl)-4-[(6bR,10aS)-2,3,6b,9,10,10a-hexahydro-3-methyl-1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]-1-butanone. Its chemical formula is C24H28FN3O, and its molecular weight is approximately 393.5 g/mol.
The mechanism of action of Lumateperone is unknown concerning schizophrenia treatment. But, the efficacy of lumateperone is mediated with a combination of postsynaptic antagonist activity at central dopamine D2 receptors and antagonist activity at central serotonin 5-HT2A receptors.
Lumateperone Impurities and Synthesis
During the synthesis1 and storage of Lumateperone, impurities may form, including degradants, process-related, and potential genotoxic impurities. Control of these impurities2 is essential to ensure the drug’s safety, quality, and efficacy. Various techniques, such as high-performance liquid chromatography (HPLC), are used to identify, quantify, and control these impurities.
Daicel Pharma issues a Certificate of Analysis (CoA) for Lumateperone impurity standards, including Desmethyl Lumateperone, Lumateperone Metabolite 308, Lumateperone Metabolite 565, Lumateperone metabolite M131, Lumateperone Metabolite M309, Lumateperone Tosylate, and Ortho Isomer of Lumateperone (Tosylate salt). The CoA is from an analytical facility that complies with current Good Manufacturing Practices (cGMP) and includes comprehensive characterization data, such as 1H NMR, 13C NMR, IR, MASS, and HPLC purity. We give additional characterization data such as 13C-DEPT and CHN on request. Daicel Pharma can prepare unknown Lumateperone impurities or degradation products and offer labeled compounds to help measure the effectiveness of Lumateperone. Additionally, we offer highly pure Lumateperone 13CD3 tosylate salt, deuterium-labeled Lumateperone standard for bioanalytical research and BA/BE studies. A complete characterization report accompanies each delivery.
References
FAQ's
References
- Robichaud, Albert J.; Lee, Taekyu; Deng, Wei; Mitchell, Ian S.; Haydar, Simon; Chen, Wenting; McClung, Christopher D.; Calvello, Emilie J. B.; Zawrotny, David M., Substituted heterocycle fused gamma-carbolines, Du Pont Pharmaceuticals Company, United States, US6548493B1, April 15, 2003
- Mates et.al, Pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxalines derivatives and [1,4]oxazino[2,3,4-hi]pyrido[4,3-b]indole derivatives, Intra-Cellular Therapies, Inc, US8993572B2, March 31, 2015
Frequently Asked Questions
How can impurities in Lumateperone be minimized?
Impurities in Lumateperone are minimized using high-quality starting materials, optimized manufacturing processes, and appropriate storage conditions. In addition, analytical methods monitor and control them.
What is the role of analytical testing in controlling Lumateperone impurities?
Analytical testing plays a critical role in controlling impurities in Lumateperone by providing information on the identity and quantity of the drug product.
Which solvent helps in the analysis of Lumateperone impurities?
Acetonitrile is a solvent used in analyzing many impurities in Lumateperone.
What are the temperature conditions required to store Lumateperone impurities?
Lumateperone impurities are stored at a controlled room temperature between 2-8 ⁰C or as indicated on the Certificate of Analysis (CoA).
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.