LOSARTAN
General Information
Losartan Impurities and Losartan
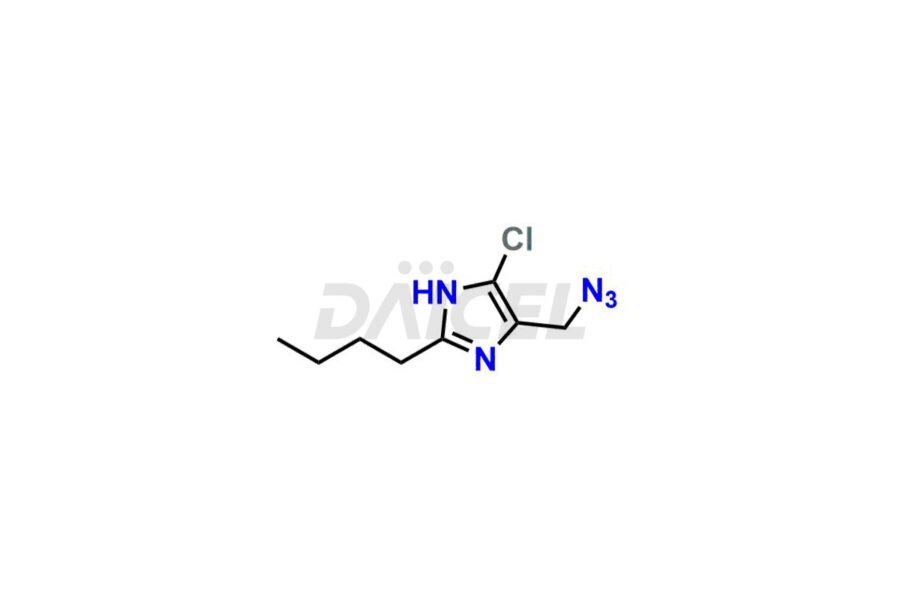
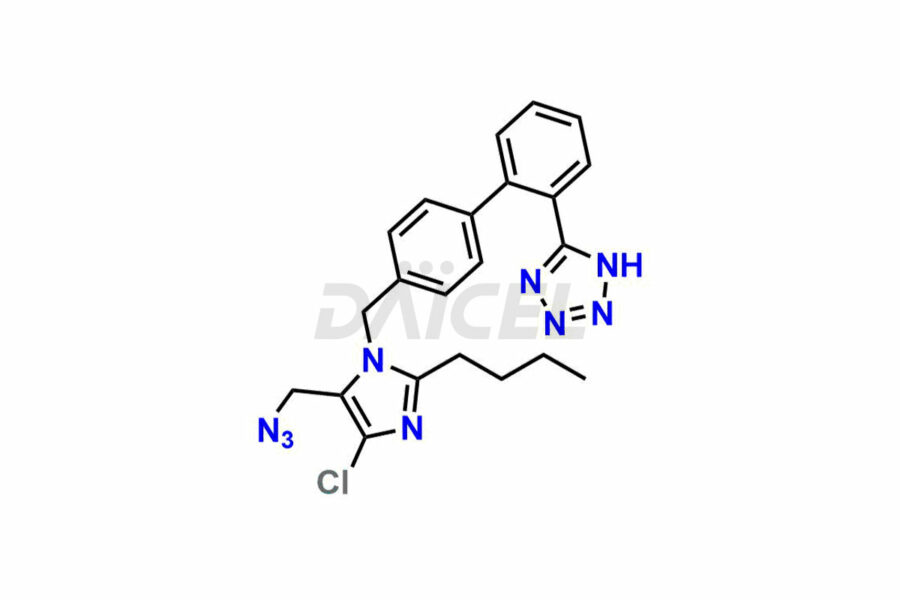
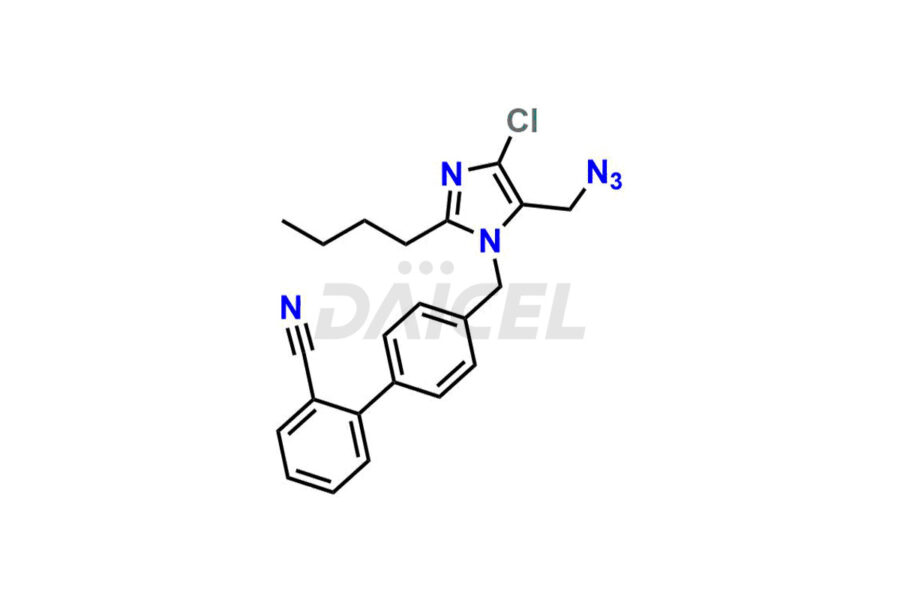
For evaluating the purity and safety of Losartan, an essential active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Losartan impurity standards. These impurity standards include crucial compounds such as 4-(azidomethyl)-2-butyl-5-chloro-1H-imidazole, Losartan azide, Losartan Azide Nitrile, and more. Additionally, Daicel Pharma provides worldwide delivery options for Losartan impurity standards.
Losartan [CAS: 114798-26-4], an effective antihypertensive medication, acts as a non-peptide antagonist of the angiotensin II receptor type 1. It treats hypertension and diabetic nephropathy.
Losartan: Use and Commercial Availability
Losartan, marketed under the brand name, Cozaar, is a US FDA-approved medication for various medical conditions. It treats hypertension and diabetic nephropathy. Losartan is an angiotensin II receptor blocker (ARB), and it has renoprotective effects in individuals with type 2 diabetes. Moreover, in cases of hypertension with left ventricular hypertrophy, Losartan inhibits angiotensin II-induced cardiac remodeling and reduces the risk of stroke in these patients.
Losartan Structure and Mechanism of Action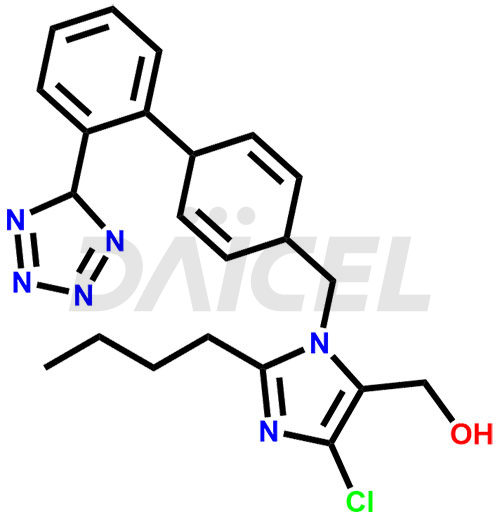
The chemical name of Losartan is 2-Butyl-4-chloro-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol. Its chemical formula is C22H23ClN6O, and its molecular weight is approximately 422.9 g/mol.
Losartan is a vasoconstrictor that blocks the binding of angiotensin II to the AT1 receptor found in many tissues.
Losartan Impurities and Synthesis
The analysis and control of impurities in Losartan1, an angiotensin II receptor blocker, is crucial to ensure the quality and safety of the medication. Various Losartan impurities include related substances and degradation products that require identification and quantification. Analytical techniques such as high-performance liquid chromatography (HPLC) and liquid chromatography (LC) analyze these impurities. Stringent control measures and specifications limit the impurity levels in Losartan, ensuring compliance with regulatory requirements.
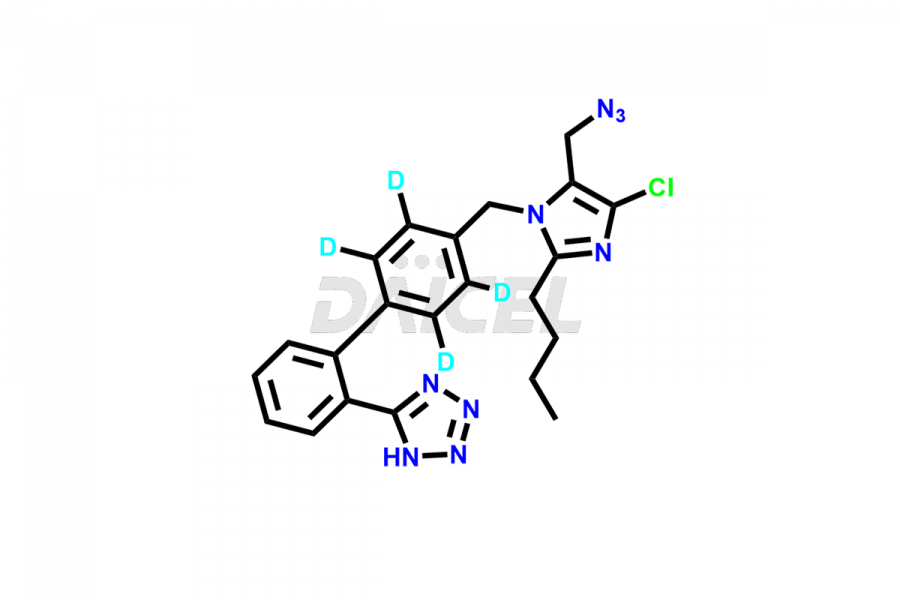
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for the preparation of Losartan impurity standards, which include 4-(azidomethyl)-2-butyl-5-chloro-1H-imidazole, Losartan azide, Losartan Azide Nitrile, and so on. In addition, we offer deuterium-labeled Losartan compound, Losartan Impurity 21-D4, which is essential for conducting bioanalytical research and BA/BE studies. Our Losartan impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Losartan impurity standards, degradation products, and labeled compounds to evaluate the effectiveness of generic Losartan. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Carini, David John; Duncia, John Jonas Vytautas, Angiotensin II receptor blocking imidazoles, du Pont de Nemours, E. I., and Co., United States, EP253310B1, October 26, 1994
- Williams, R. C.; Alasandro, M. S.; Fasone, V. L.; Boucher, R. J.; Edwards, J. F., Comparison of liquid chromatography, capillary electrophoresis and supercritical fluid chromatography in the determination of Losartan Potassium drug substance in Cozaar tablets, Journal of Pharmaceutical and Biomedical Analysis, Volume: 14, Issue: 11, Pages: 1539-1546, 1996
Frequently Asked Questions
Can impurity control measures vary based on the intended use of Losartan?
Impurity control measures may vary depending on the dosage form and the intended use of Losartan, such as oral tablets, suspensions, or injectables. Each formulation may have its impurity limits and specifications.
How are Losartan impurities controlled during the manufacturing process?
Manufacturing processes for Losartan include stringent controls such as good manufacturing practices (GMP) and quality control measures to minimize impurities at every stage, from raw material selection to final product packaging.
Which solvent helps in analyzing Losartan impurities?
Methanol and Acetonitrile are solvents when analyzing many impurities in Losartan.
What is the recommended storage temperature for Losartan impurities?
Losartan impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.