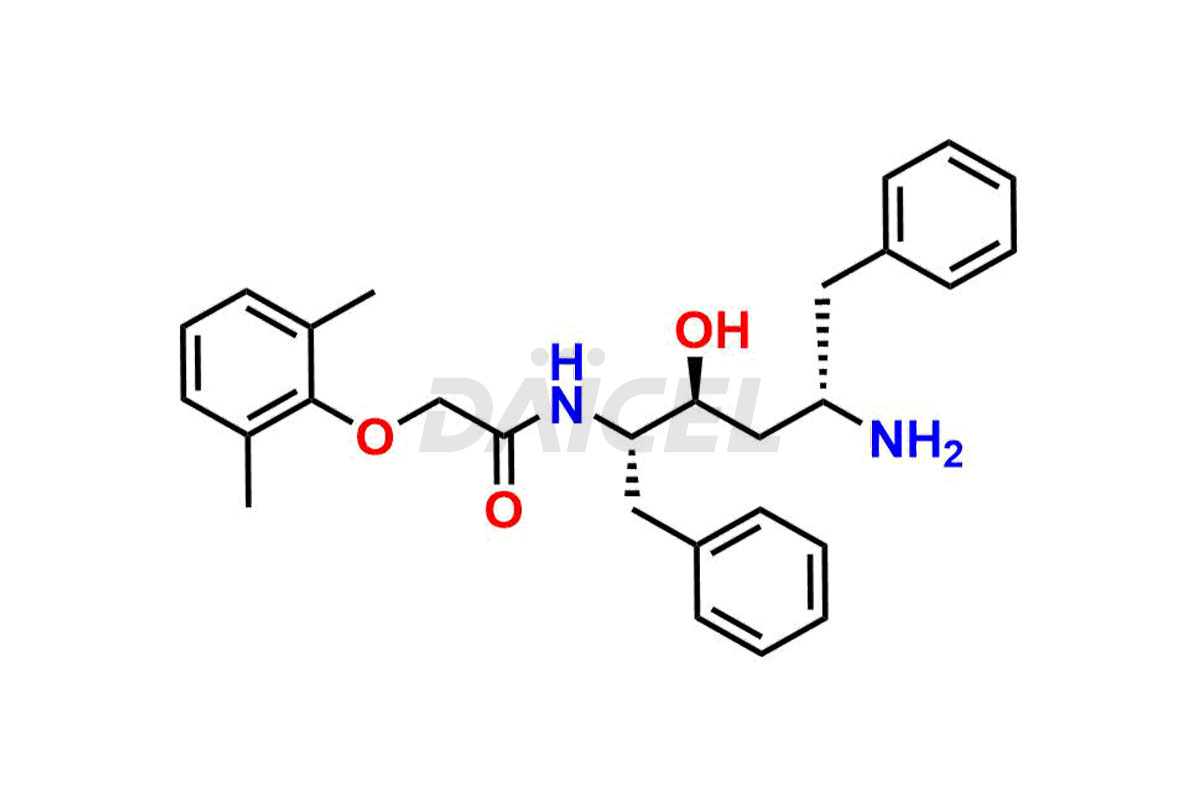
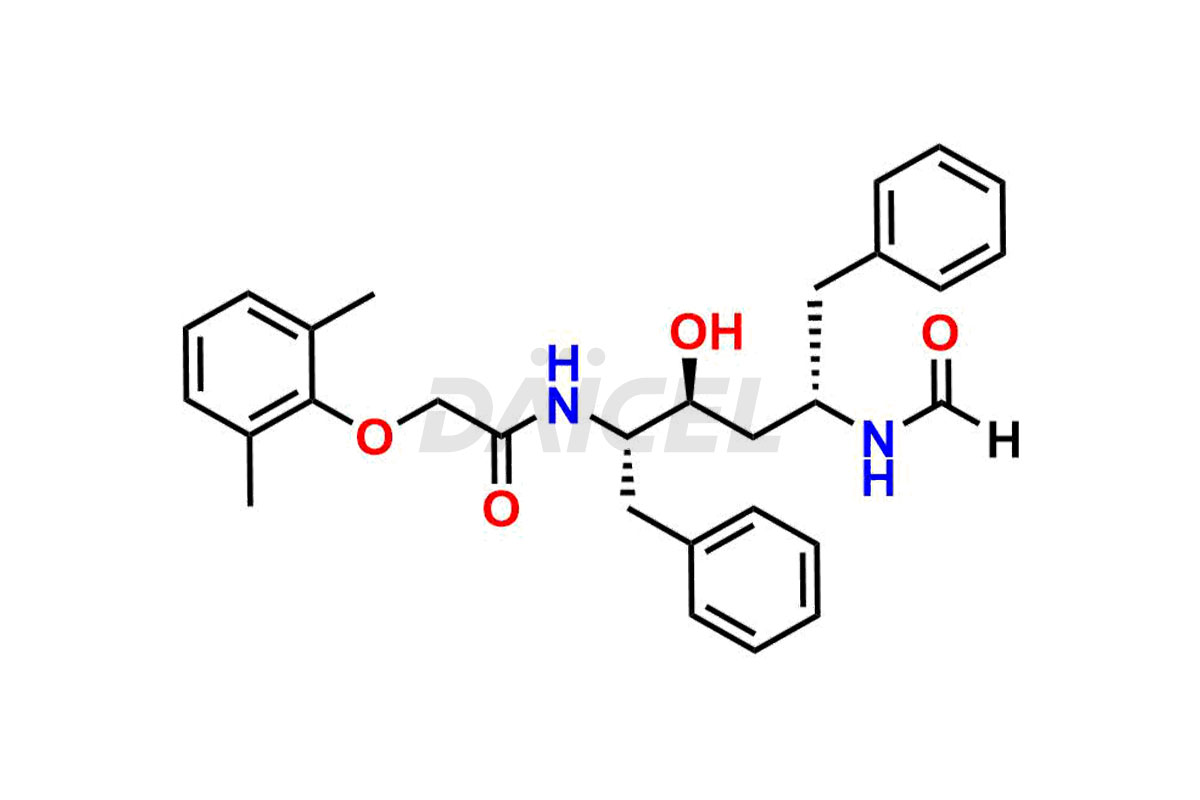
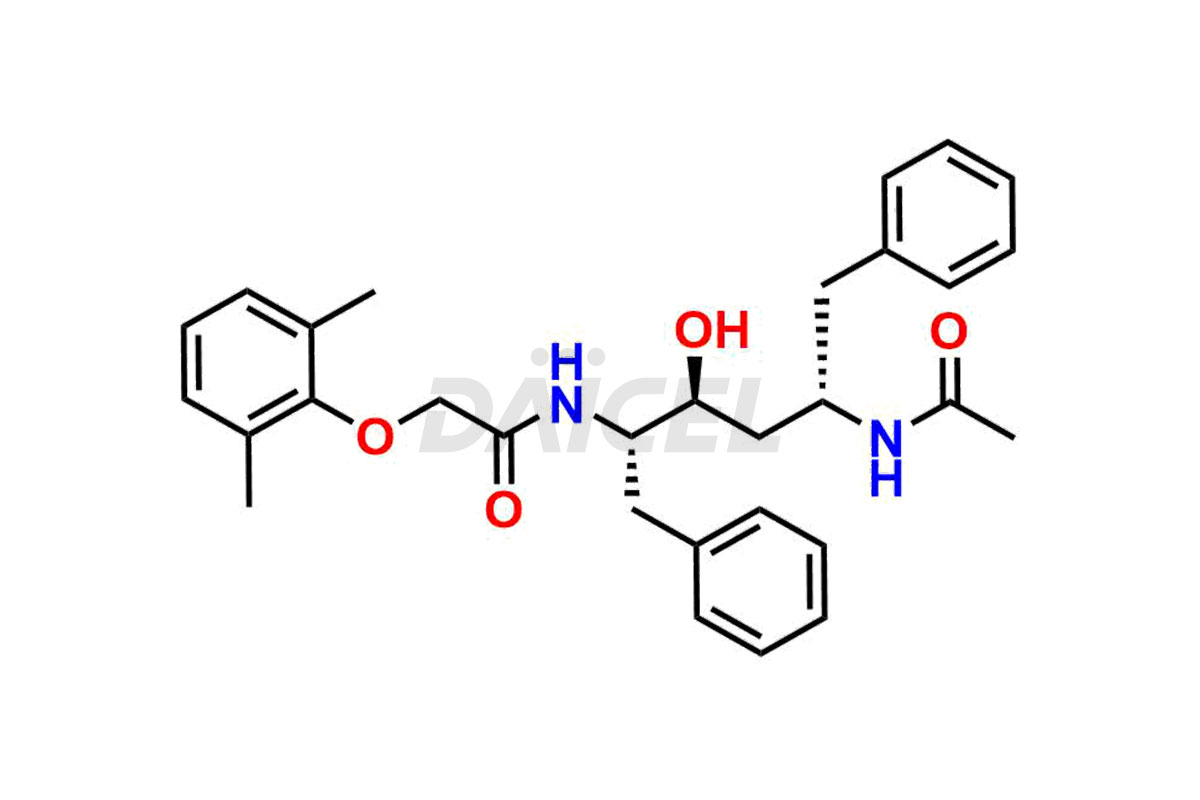
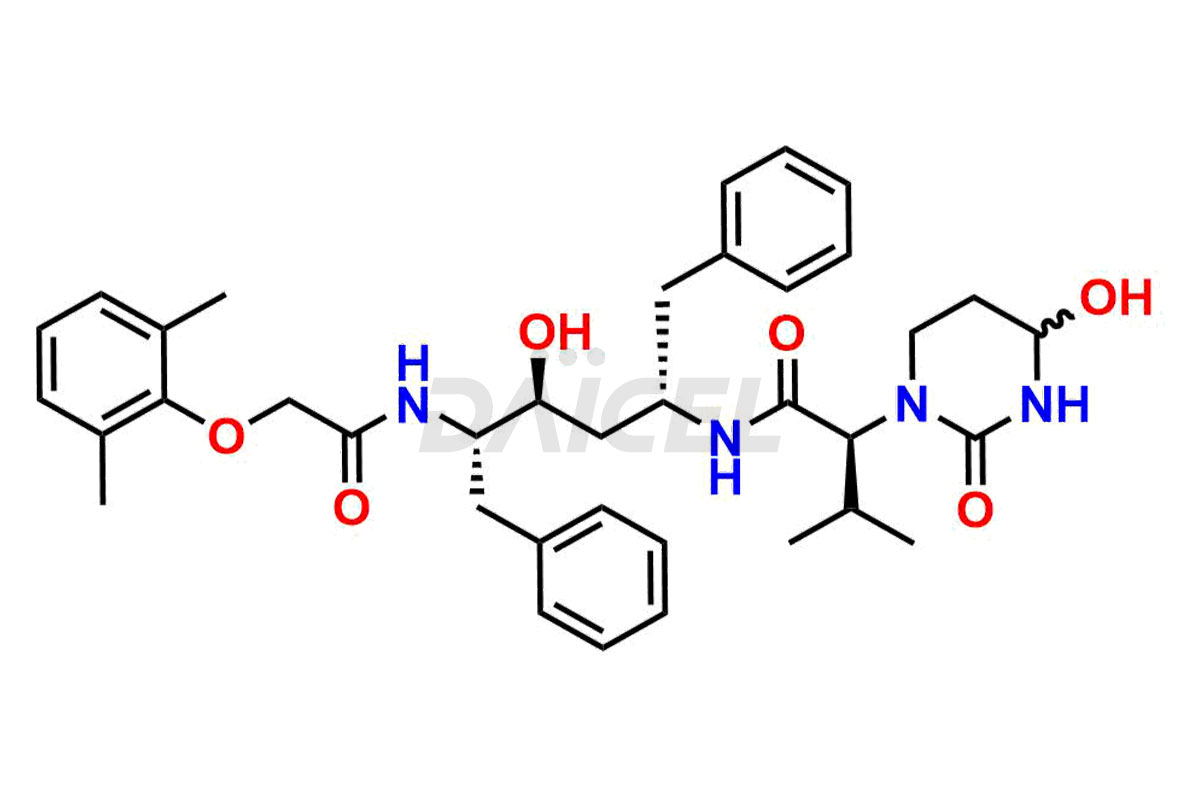
Lopinavir
General Information
Lopinavir Impurities and Lopinavir
For evaluating the purity and safety of Lopinavir, an essential active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Lopinavir impurity standards. These impurity standards include crucial compounds such as Lopinavir EP Impurity-E, Lopinavir EP Impurity-F, Lopinavir EP Impurity-G, Lopinavir Metabolite M1 Impurity, and Lopinavir Metabolite M3-M4 Impurity. Additionally, Daicel Pharma provides worldwide delivery options for Lopinavir impurity standards.
Lopinavir [CAS: 192725-17-0] is an antiretroviral protease inhibitor in combination with ritonavir for treating and preventing HIV infection and AIDS. It is a peptidomimetic HIV protease inhibitor that remains effective against HIV protease even in the presence of the Val 82 mutation.
Lopinavir: Use and Commercial Availability
Kaletra is the brand name for the combination product Lopinavir/Ritonavir, which combines with antiretrovirals and treats HIV-1 infection in adults and pediatric patients ≥14 days old. This product consists of an HIV protease inhibitor, Lopinavir, and the inhibitor of cytochrome P-450 CYP3A, Ritonavir.
Lopinavir Structure and Mechanism of Action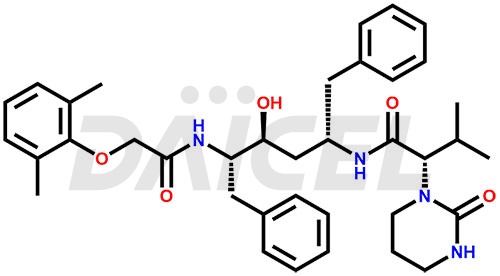
The chemical name of Lopinavir is (αS)-N-[(1S,3S,4S)-4-[[2-(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide. Its chemical formula is C37H48N4O5, and its molecular weight is approximately 628.8 g/mol.
Lopinavir prevents the cleavage of the Gag-Pol polyprotein. It inhibits the preparation of immature, non-infectious viral particles.
Lopinavir Impurities and Synthesis
Impurities in Lopinavir are unintended substances that can be present in the drug product with the active pharmaceutical ingredient (API). They may originate from manufacturing1, starting materials, or interactions with environmental factors. Common Lopinavir impurities include related compounds, degradation products, residual solvents, and process-related impurities. It is crucial to monitor and control the levels of these impurities during manufacturing to ensure the safety, efficacy, and quality of the Lopinavir drug product. Regulatory authorities have established guidelines and limits for impurity levels to maintain purity and minimize potential risks associated with the drug. Stringent quality control measures minimize impurity levels and ensure product integrity.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Lopinavir impurity standards, which include Lopinavir EP Impurity-E, Lopinavir EP Impurity-F, Lopinavir EP Impurity-G, Lopinavir Metabolite M1 Impurity, and Lopinavir Metabolite M3-M4 Impurity. Our Lopinavir impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Lopinavir impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Sham, Hing Leung; Norbeck, Daniel W.; Chen, Xiaoqi; Betebenner, David A.; Kempf, Dale J.; Herrin, Thomas R.; Kumar, Gondi N.; Condon, Stephen L.; Cooper, Arthur J.; Dickman, Daniel A.; et al, Retroviral protease inhibiting compounds, Abbott Laboratories, United States, US5914332A, June 22, 1999
- Faux, J.; Venisse, N.; Olivier, J. C.; Bouquet, S., Rapid high-performance liquid chromatography determination of lopinavir, a novel HIV-1 protease inhibitor, in human plasma, Chromatographia, Volume: 54, Issue: 7/8, Pages: 469-473, 2001
Frequently Asked Questions
Are Lopinavir impurities a concern for drug interactions?
Some impurities in Lopinavir, particularly related compounds, may interact with other drugs, leading to drug-drug interactions. Proper monitoring and control of impurity levels help minimize the risk of such interactions and ensure the drug's safety and effectiveness.
Can Lopinavir impurities impact its bioequivalence to other formulations?
Impurities in Lopinavir can potentially affect its bioequivalence to other formulations. Regulatory agencies require that generic formulations demonstrate comparable impurity profiles and bioequivalence to the reference product to ensure therapeutic equivalence and patient safety.
What solvent is typically used for analyzing Lopinavir impurities?
Methanol and Acetonitrile are solvents used when analyzing many impurities in Lopinavir.
What is the recommended storage temperature for Lopinavir impurities?
Lopinavir impurities should be stored, at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.