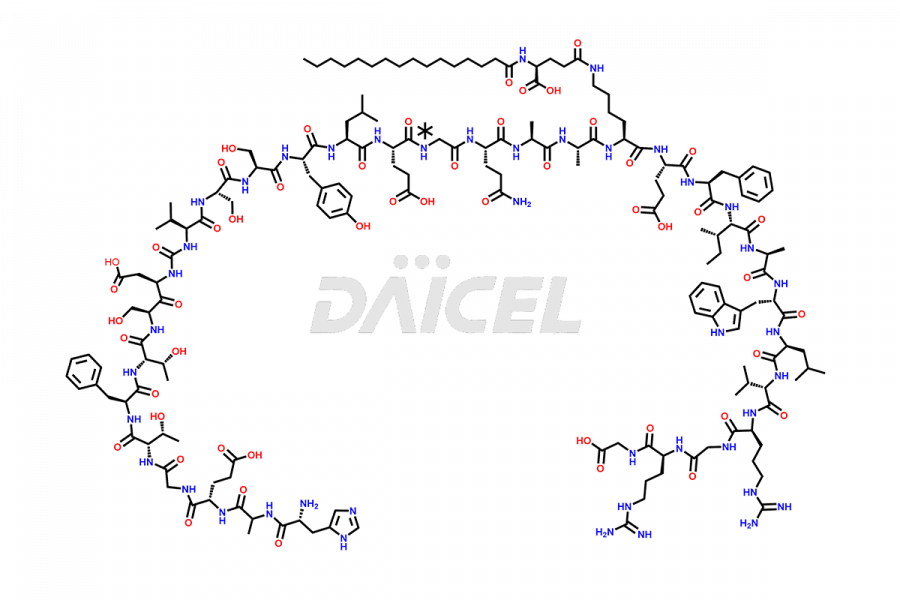
Liraglutide
References
- Victoza-Product monograph-Novo Nordisk
- Liselotte Bjerre Knudsen, Per Olaf Huusfeldt, Per Franklin Nielsen, Niels C. Kaarsholm, Helle Birk Olsen, S0ren Erik Bj0rn, Freddy Zimmerdahl Pedersen, Kjeld Madsen; Novo Nordisk A/S, “Derivatives of GLP-1 analogs”, Denmark, US patent, US 6,268,343 Bl, July 31, 2001
- Shah, Mubarak Hasan; Reddy, Padmanabha Reddy Yeragam; Bhawanishankar, Madhira; Raja, Mokkaisamy Jegadeesh; Pillai, Manoj,” UHPLC-MS/MS determination of GLP-1 analogue, liraglutide a bioactive peptide in human plasma”, European Journal of Biomedical and Pharmaceutical Sciences, Volume: 4, Issue: 3, Pages: 304-311, 2017
Frequently Asked Questions
How are Liraglutide impurities identified?
Liraglutide impurities can be identified using analytical methods such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), etc.
How are Liraglutide impurities purified after synthesis?
Liraglutide impurities can be purified after synthesis using various techniques such as preparative HPLC, flash chromatography, or crystallization. These methods involve separating the impurities from the main product based on their chemical properties and physical characteristics.
What are the temperature conditions required to store Liraglutide impurities?
Liraglutide impurities should be stored at recommended temperature conditions, typically 2-8°C or -20°C, depending on their stability. Improper storage can lead to degradation and loss of purity, so it's important to follow the manufacturer's instructions.
Why are Liraglutide impurities essential in drug development?
Liraglutide impurities can affect drug efficacy and safety. It is observed that impurities can cause adverse effects such as allergic reactions, injection site reactions, gastrointestinal disturbances, pancreatitis, or liver damage due to their toxicity or carcinogenic nature. Identifying and quantifying the impurities is essential during drug development.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.