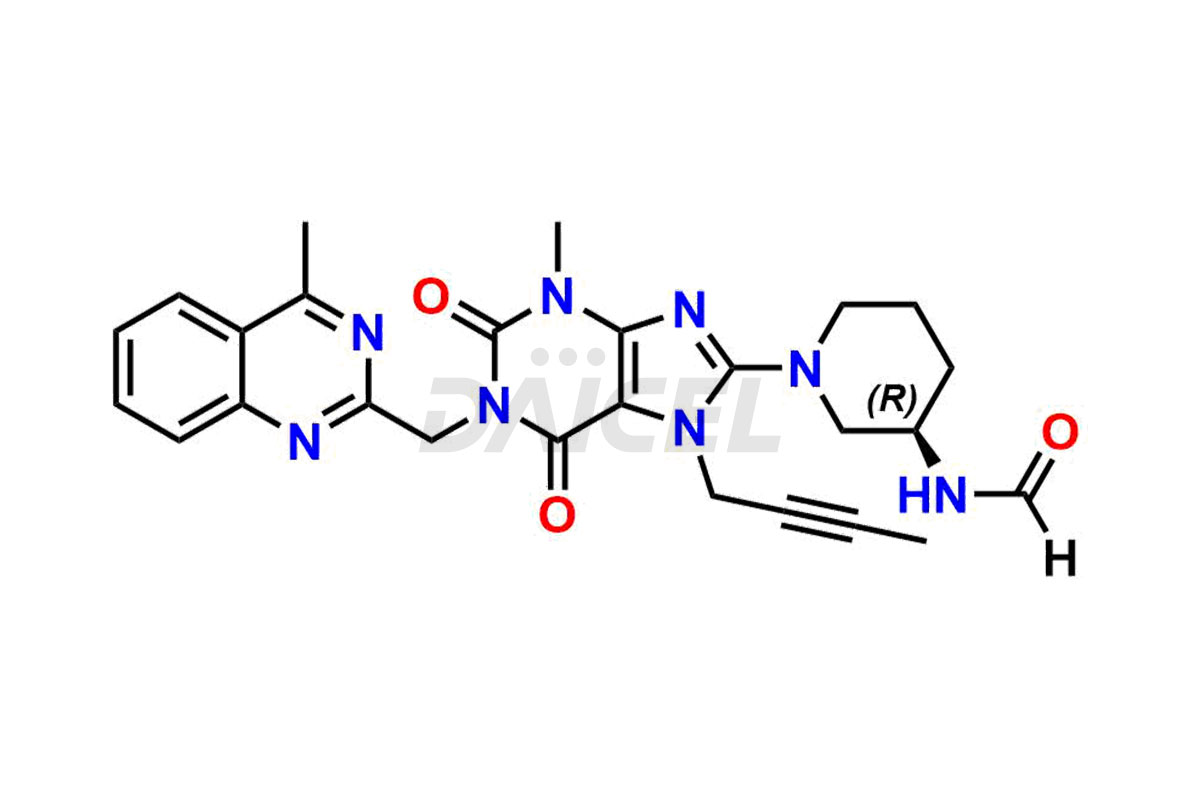
Linagliptin
References
- Himmelsbach, Frank; Langkopf, Elke; Eckhardt, Matthias; Mark, Michael; Maier, Roland; Lotz, Ralf Richard Hermann; Tadayyon, Mohammad,8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions, Boehringer Ingelheim Pharma G.m.b.H. & Co. K.-G., Germany, US7407955B2, August 5, 2008
- El-Bagary, Ramzia Ismail; Elkady, Ehab Farouk; Ayoub, Bassam Mahfouz, Spectrofluorometric determination of linagliptin in bulk and in pharmaceutical dosage form,European Journal of Chemistry, Volume: 5, Issue: 2, Pages: 380-382, 3 pp., 2014
Frequently Asked Questions
Are there any specific guidelines or requirements for reporting Linagliptin impurities?
Regulatory guidelines outline the necessary documentation and reporting of Linagliptin impurities to ensure transparency and accountability.
Are Linagliptin impurities tested for their genotoxic potential?
Genotoxicity testing is typically conducted to assess the potential of Linagliptin impurities to cause DNA damage or mutations.
Can Linagliptin impurities result from inadequate manufacturing processes?
Improper manufacturing processes, such as poor handling or contamination, can contribute to the formation of impurities in Linagliptin.
What is the recommended storage temperature for Linagliptin impurities?
Linagliptin impurities should be stored at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.