Lifitegrast
General Information
Lifitegrast Impurities and Lifitegrast
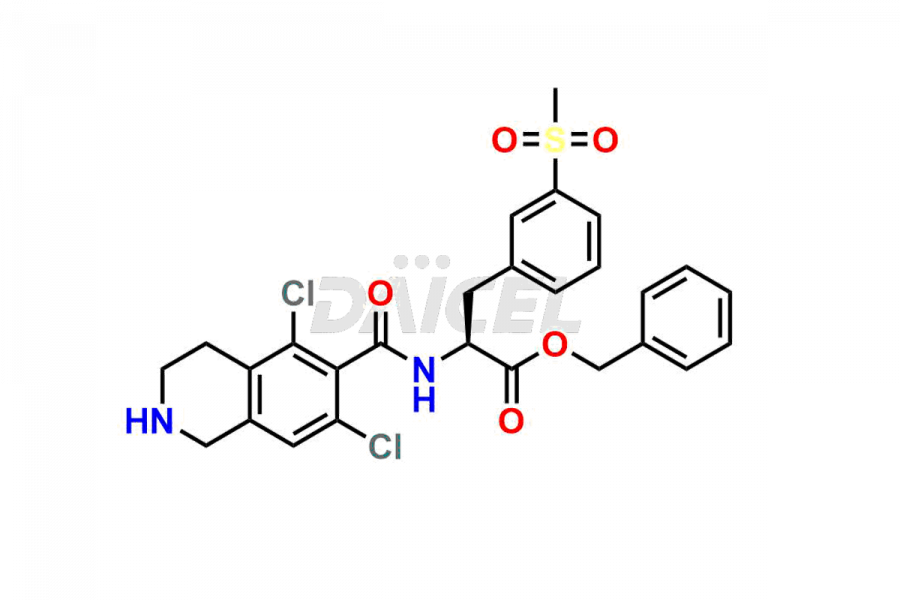
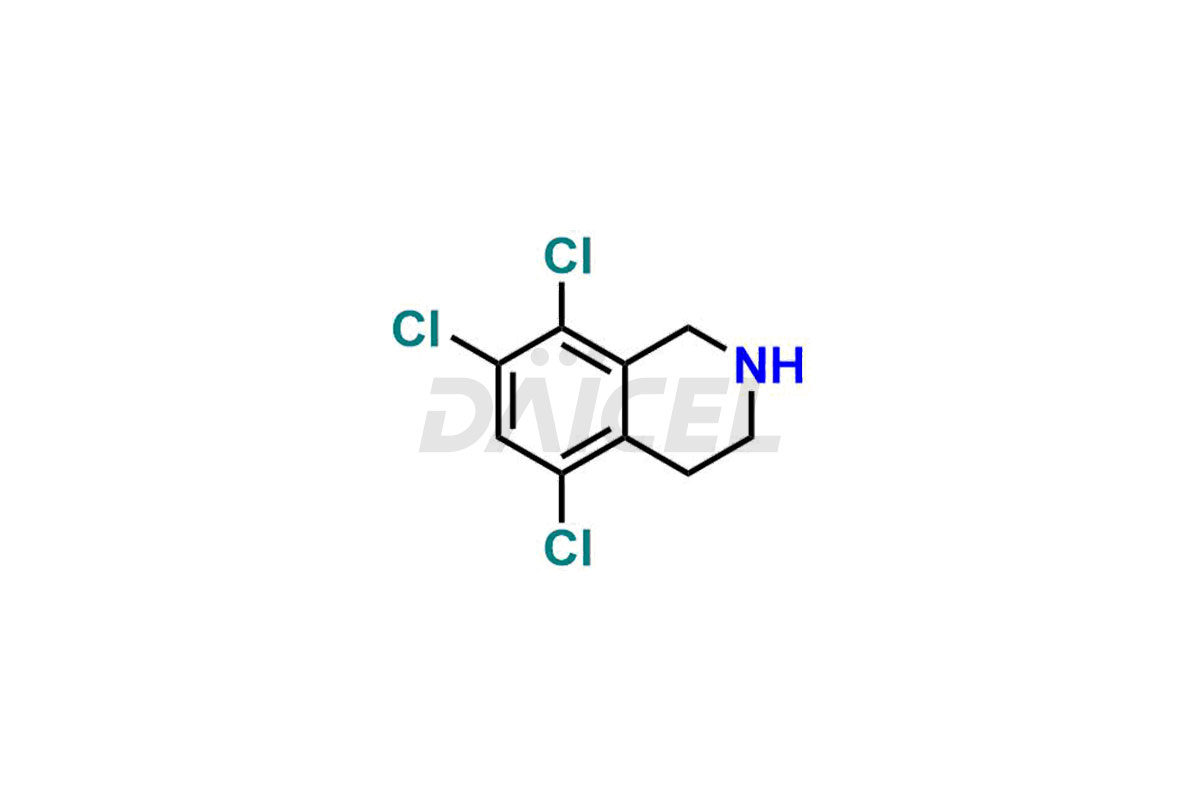
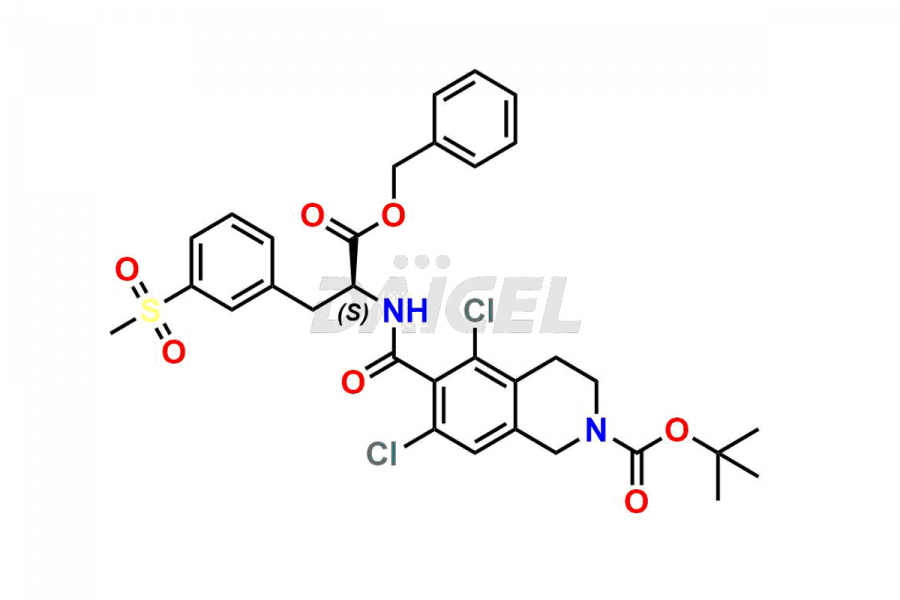
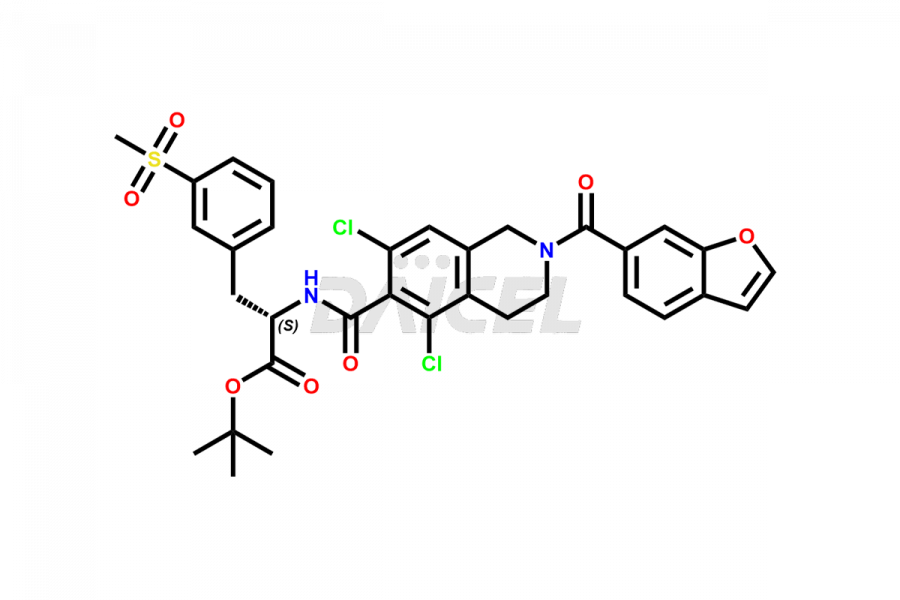
For the purity and safety of Lifitegrast, an active pharmaceutical ingredient, Daicel Pharma offers a customized synthesis of Lifitegrast impurity standards. These impurity standards include crucial compounds such as (S)-benzyl 2-(5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3 -(3-(methylsulfonyl)phenyl)propanoate, 5,7,8-trichloro-1,2,3,4-tetrahydroisoquinoline, Lifitegrast Impurity 1, Lifitegrast Impurity 3, Lifitegrast Impurity 5, Lifitegrast R-Isomer, and Lifitegrast tertiary butyl ester impurity. Additionally, Daicel Pharma provides worldwide delivery options for Lifitegrast impurity standards.
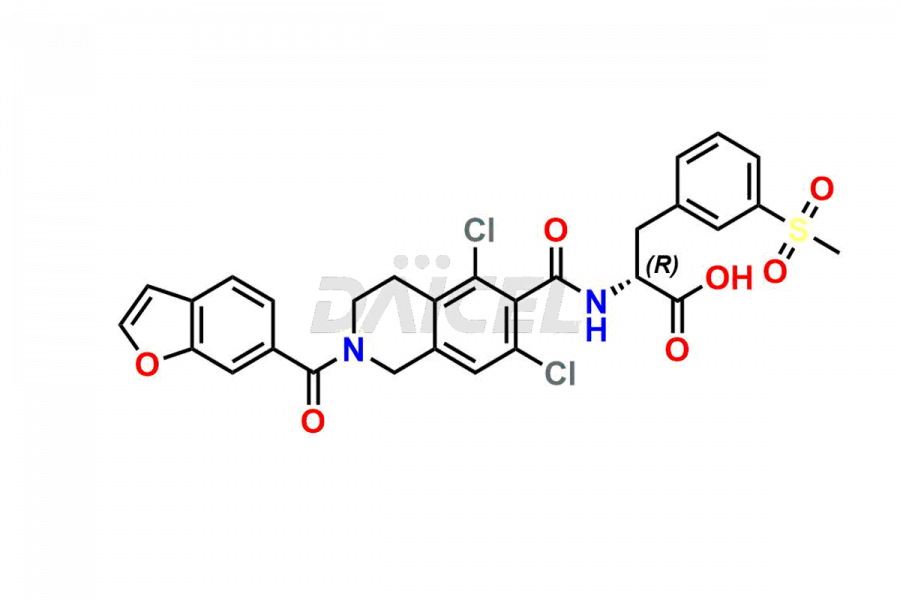
Lifitegrast [CAS: 1025967-78-5] is an anti-inflammatory drug and a lymphocyte function-associated antigen-1 antagonist for treating keratoconjunctivitis sicca, also known as dry eye syndrome.
Lifitegrast: Use and Commercial Availability
Lifitegrast, marketed as Xiidra, is a topical anti-inflammatory agent approved by the US FDA for treating dry eye syndrome. It acts as a small lymphocyte function-associated antigen-1 (LFA-1) antagonist, reducing inflammation and managing dry eye symptoms. Lifitegrast is administered as one drop of a 5% ophthalmic solution and used for artificial tears.
Lifitegrast Structure and Mechanism of Action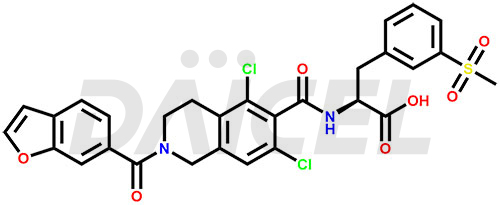
The chemical name of Lifitegrast is N-[[2-(6-Benzofuranylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl]carbonyl]-3-(methylsulfonyl)-L-phenylalanine. Its chemical formula is C29H24Cl2N2O7S, and its molecular weight is approximately 615.5 g/mol.
Lifitegrast blocks the interaction of lymphocyte function-associated antigen-1 (LFA-1) with its cognate ligand intercellular adhesion molecule-1 (ICAM-1).
Lifitegrast Impurities and Synthesis
Lifitegrast impurities are unintended substances that may be present in Lifitegrast formulations. They can occur during the drug’s synthesis1, storage, or transportation. It is essential to closely monitor and control impurity levels in Lifitegrast to ensure its safety, efficacy, and quality. Pharmaceutical manufacturers employ rigorous quality control measures to minimize impurity levels and maintain the purity of Lifitegrast. By adhering to strict guidelines and regulatory standards, companies strive to provide patients with reliable and effective Lifitegrast formulations for treating dry eye syndrome. Continuous monitoring and testing help ensure that impurities are kept at acceptable levels, maintaining their integrity throughout their shelf life.
Daicel Pharma strictly adheres to cGMP standards and operates an analytical facility for preparing Lifitegrast impurity standards, which include (S)-benzyl 2-(5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3 -(3-(methylsulfonyl)phenyl)propanoate, 5,7,8-trichloro-1,2,3,4-tetrahydroisoquinoline, Lifitegrast Impurity 1, Lifitegrast Impurity 3, Lifitegrast Impurity 5, Lifitegrast R-Isomer, and Lifitegrast tertiary butyl ester impurity. Our Lifitegrast impurity standards have a detailed Certificate of Analysis (CoA) that provides a comprehensive characterization report. This report includes data obtained through techniques, 1H NMR, 13C NMR, IR, MASS, and HPLC purity analysis2. Upon request, we give additional data like 13C-DEPT. Moreover, we can synthesize unknown Lifitegrast impurity standards and degradation products. Each delivery has a comprehensive characterization report.
References
FAQ's
References
- Gadek, Thomas; Burnier, John, Compositions And Methods For Treatment Of Eye Disorders, Sarcode, United States, EP1881823B1, December 3, 2014
- Kumar, Ajay; Chalannavar, Raju Krishna, Characterization of Degradation Products of Lifitegrast by Mass Spectrometry: Development and Validation of a Stability-indicating Reversed Phase HPLC Method, Analytical Chemistry Letters, Volume: 12, Issue: 6, Pages: 730-744, 2022
Frequently Asked Questions
Do impurity profiles in Lifitegrast vary between different batches?
While manufacturers strive to maintain consistent impurity profiles, minor variations in impurity levels may occur between batches of Lifitegrast.
Can Lifitegrast impurities impact the bioavailability of the drug?
Some impurities may interfere with the absorption, distribution, or metabolism of Lifitegrast, potentially affecting its bioavailability and therapeutic effects.
Are there any Lifitegrast impurities that can result from the degradation of excipients?
The degradation of excipients used in Lifitegrast can lead to the formation of impurities, which need to be carefully controlled and monitored.
What is the recommended storage temperature for Lifitegrast impurities?
Lifitegrast impurities should be stored at a controlled room temperature, usually between 2-8 °C.
Note: Products protected by valid patents by a manufacturer are not offered for sale in countries having patent protection. The sale of such products constitutes a patent infringement, and its liability is at the buyer's risk.